Figure 3.
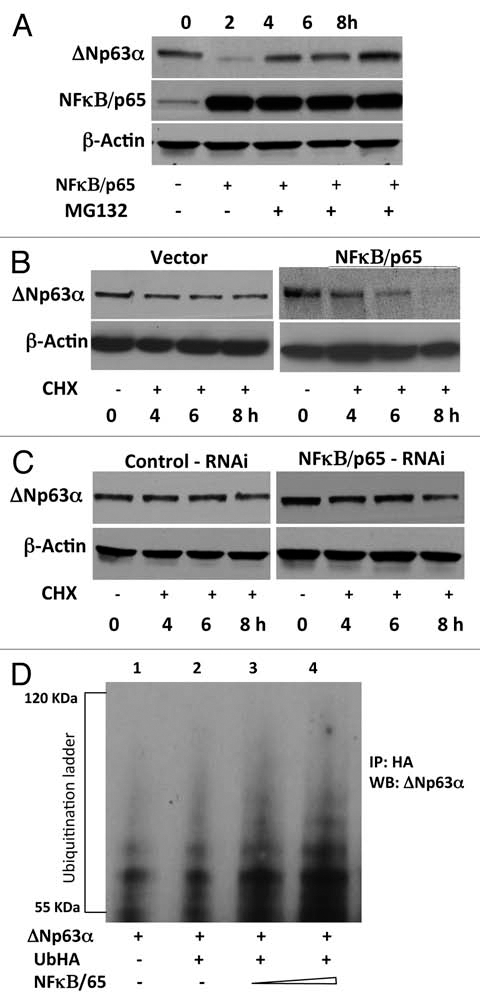
NFκB/p65 promotes ubiquitin-mediated proteasomal degradation of ΔNp63α. (A) JHU-022 cells were transfected with or without an expression plasmid encoding NFκB/p65; 24 h after transfection, cells were treated with 10 µM MG132 for the indicated time periods. Whole cell lysates were subjected to western Blot analysis using anti-NFκB/p65 or anti-p63 antibodies. (B) JHU-022 cells were transfected with constant amount of ΔNp63α expression plasmid with or without expression plasmids encoding NFκB/p65, or empty vector, as indicated. 24 h after transfection, the cells were treated with 100 µg/ml cycloheximide. At the indicated time points, whole cell lysates were analyzed for ΔNp63α by immunoblotting. Actin was used for loading control. (C) JHU-022 cells were transfected with constant amount of NFκB/p65 RNAi expression plasmid. 24 h after transfection, the cells were treated with 100 µg/ml cycloheximide. At the indicated time points, whole cell lysates were analyzed for endogenous ΔNp63α by immunoblotting. Actin was used for loading control. (D) JHU-022 cells were co-transfected with ΔNp63α and Ub-HA expression plasmids, with or without increasing concentrations of an expression vector encoding NFκB/p65. At 36 h following transfection, cells were treated with MG132 for 10 h. Cell lysates were immunoprecipitated with anti-HA-matrix and subjected to western blot analysis with an antibody that recognizes ΔNp63α to assess the ubiquitination levels of ΔNp63α.
