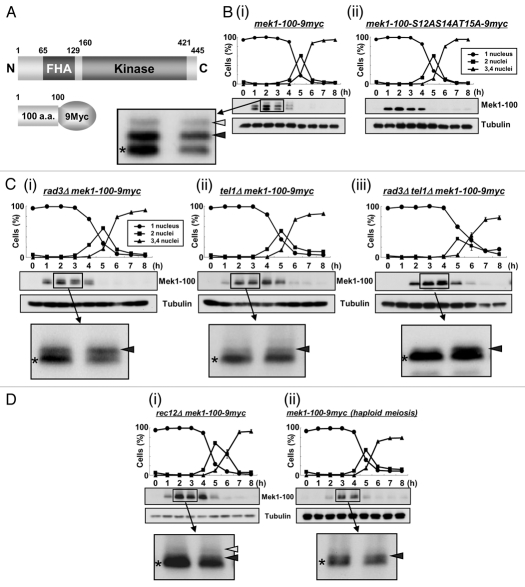
Figure 2.
Mek1 is phosphorylated in vivo during meiosis. (A) Schematic of the truncated Mek1-100-9Myc protein fusion. Plasmids carrying the Mek1-100-9Myc fusion construct or a construct with the S12AS14AT15A mutations were integrated into the mek1 promoter locus in mek1Δ cells. (B) Detection of Mek1 bands in mek1-100-myc cells (TT623) (i) or mek1-100-S12AS14AT15A-9myc cells (TT710) (ii) during meiosis by western blot analysis. Only a single band is detected in TT710 cells, in which S12, S14 and T15 of Mek1 are replaced by alanine. (C) Detection of multiple bands of Mek1 in rad3Δ mek1-100-9myc cells (TT705) (i), tel1Δ mek1-100-9myc cells (TT286) (ii) or rad3Δ tel1Δ mek1-100-9myc cells (TT201) (iii) during meiosis. Asterisks indicate putative unphosphorylated bands, while white and black arrowheads denote putative phosphorylated bands. The intensity of the black arrowhead band is remarkably weakened in tel1Δ mek1-100-9myc cells, suggesting it to be primarily derived from phosphorylation by Tel1. (D) Detection of multiple bands of Mek1 during meiosis in rec12Δ mek1-100-9myc cells (TT266) (i) or in haploid mek1-100-9myc cells (TT608) (ii). The rec12Δ cells cannot generate double strand breaks during meiosis, while the mek1-100-9myc cells were induced to undergo haploid meiosis in the pat1 genetic background. (E) Hydroxyurea (HU) blocks DNA replication of mek1-100-9myc cells, as observed by the persistence of single-nucleus cells and by FACS analysis. (F) Detection of multiple Mek1 bands in mek1-100-9myc cells after HU treatment during meiosis.