Figure 1.
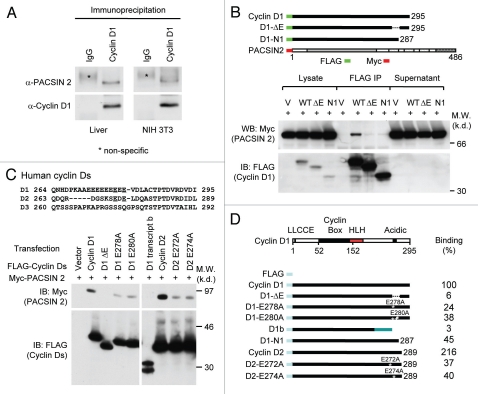
Cyclin D1 binds to PACSIN 2 through its C-terminal E-rich motif. (A) Immunoprecipitation (IP)-western blot (WB) was performed to determine the binding of endogenous cyclin D1 and Pacsin 2. Protein lysates were prepared from either NIH 3T3 cells or murine liver tissue. IP was conducted with a cyclin D1 antibody pre-conjugated to agarose. The immunoprecipitates was subjected to WB for detection of Pacsin 2. (B) IP-WB was conducted of cells transfected with either an expression vector for Myc epitope-tagged PACSIN 2 and FLAG-tagged cyclin D1 wild type or mutant expression vectors. The schematic representation shows the acidic-rich region. The C-terminal E-rich motif is required for PACSIN 2 binding. (C) Amino acid sequence of the carboxyl terminal region of cyclin D1. IP-WB of PACSIN 2 association with D-type cyclins, wild type or mutant is as shown. IP was conducted with the FLAG antibody directed to the N-terminus of cyclin Ds with western blot to detect the Myc epitope of PACSIN 2. The amino acid residues required for the binding were mapped to E278 and E280 for cyclin D1 and E272 and E274 for cyclin D2, respectively. (D) Schematic representation of constructs of D-type cyclins that were used for mapping the domain requirement for PACSIN 2 binding.