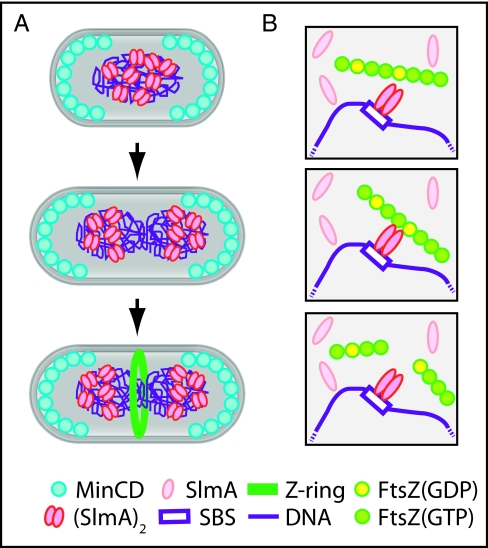
Fig. 5.
Model for FtsZ regulation by SlmA. (A) In newborn cells, MinCD and SlmA prevent Z-ring assembly at the cell poles and over the nucleoid, respectively. For simplicity, MinCD oscillation is not shown. As the chromosome is replicated, SlmA bound to chromosomal SBSs segregates with the origin-proximal regions of the daughter chromosomes. This leaves the midcell zone free of inhibitors so that the Z ring can form as replication nears completion. (B) SlmA free in the cytoplasm is monomeric and inactive. Binding to SBSs stimulates the ability of SlmA to interact with FtsZ polymers. Upon associating with polymers, it promotes their breakdown. We propose it does so by disrupting FtsZ–FtsZ interactions at interfaces where GTP has been hydrolyzed to GDP. In our model, DNA binding activates SlmA in part by promoting dimerization. It is also possible that higher order oligomers are formed and that they further enhance SlmA activity.