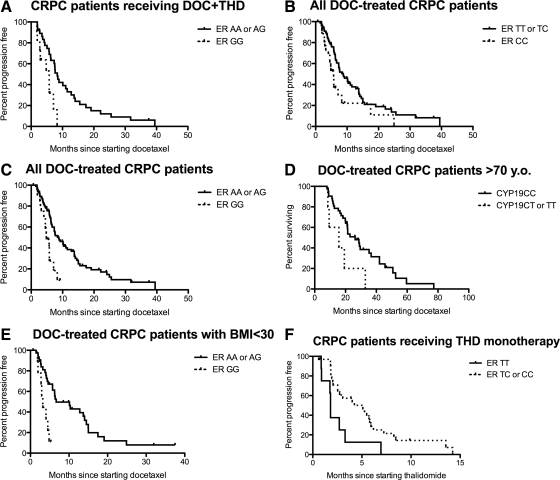
Fig. 1.
Survival characteristics of patients on the studied trials vs. genetic variants in ERα and CYP19. A, PFS of docetaxel plus thalidomide in patients carrying only ERα A>G variants (5.8 months; n = 11) and those carrying at least a single wild-type allele (8.3 months; n = 37, P = 0.015). B, PFS of combined docetaxel-based trials in patients carrying only ERα T>C variants (5.7 months; n = 26) and those carrying at least a single wild-type allele (8.8 months; n = 81; P = 0.036). C, Patients carrying only ERα A>G variants (4.8 months; n = 20) and those carrying at least a single wild-type allele (8.8 months; n = 88; P = 0.0012). D, Survival after docetaxel treatment of combined docetaxel trials in patients who were >70 years of age carrying CYP19 variant alleles (15.7 months; n = 5) and those carrying only wild-type alleles (26.3 months; n = 42; P = 0.041). E, PFS of docetaxel-based trials in individuals who were not obese (BMI < 30) carrying only ERα A>G variant alleles (3.2 months; n = 9) and those carrying at least one copy of a wild-type allele (6.7 months, n = 40; P = 0.0078). F, PFS of thalidomide monotherapy in patients carrying ERα T>C homozygous and heterozygous genotypes (5.3 and 3.1 months, respectively; n = 9 and n = 23, respectively) and those carrying wild-type alleles (1.8 months; n = 8; P = 0.040).