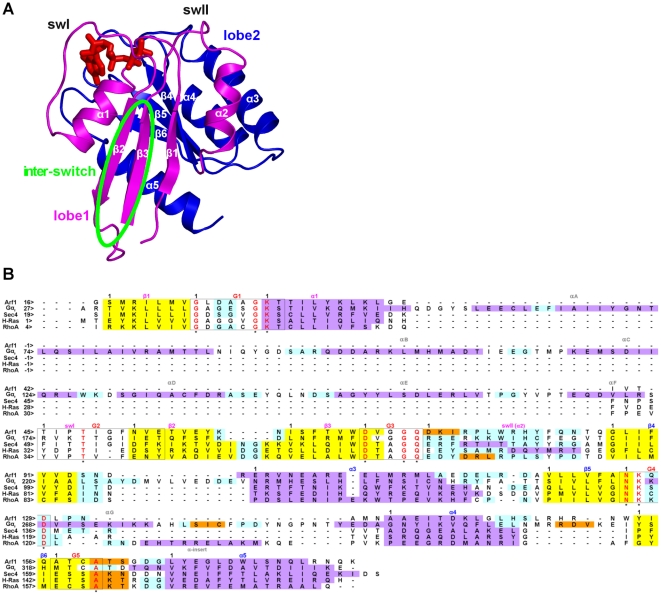
Figure 1. Structure and sequence features of the five GTPases.
A: cartoons of the H-Ras structure (PDB code: 5P21) in its GTP-bound state are shown. The Ras superfamily GTPases share a common domain, the Ras-like domain. The latter, according to CATH [34], is characterized by a Rossmann fold with a 3-layer(αβα) sandwich architecture, where helices 1 and 5 (α1 and α5; the secondary structure elements in the Ras-like domain are labeled according to the Noel's nomenclature [35]) lay on one side, whereas α2, α3, and α4 lay on the other side of the central five-stranded parallel β-sheet (i.e. comprising the β1 and β3-β6 strands, Figures 1 and 2). The helices α1 and α3 lay on the opposite side of the sheet due to the inversion in the order of the preceding strands, β1 and β3, respectively, which are adjacent to each other. The β1/α1 loop, i.e. phosphate binding loop (P loop), and the region comprising α2 as well as the preceding and following loops (i.e. switch II (swII)) participate in the binding of the nucleotide phosphates (Figures 1 and 2). The architecture of this superfamily is such that β1 is also adjacent to β4. The β1/β4 interface divides the Ras-like domain into two lobes: i) lobe 1 (i.e. the N-terminal half of the domain, magenta) includes the β1-β3 strands, the P-loop and the two switches, and ii) lobe 2 (blue), which includes the β4-β6 strands and the α3-α5 helices. Another structural feature of the conserved Ras domain is that β2 forms a β-hairpin with β3, the loop that connects the two antiparallel strands being directed towards the opposite side of the nucleotide binding cleft (Figures 1 and 2) [3]. The β2/β3 hairpin is also called “inter-switch” (i.e. delimited by a green oval) because the loops that enter β2 and exit from β3 constitute, respectively, the swI and swII regions. The loops connected to the C-term of β1 and the N-term of β2, P loop and swI, respectively, define most of the nucleotide binding site. The members of the Gα family hold an extra-Ras α-helical domain constituted by a long central helix surrounded by five shorter helices. The interface between α-helical and Ras-like domain constitutes the nucleotide binding cleft. Incidentally, among the small G proteins RhoA has a structural peculiarity consisting of a ten amino acid α-helical insertion (α-insert) on the β5/α4 loop like the αG segment shared by the members of the Gα family. B): the multiple sequence alignment derived from the multiple structure alignment of representatives of the SGTP state of Arf1 (PDB code: 1O3Y), Gαt (PDB code: 1TND), Sec4 (PDB code: 1G17), H-Ras (PDB code: 5Q21), and RhoA (PDB code: 1KMQ) is shown (i.e. achieved by the Multiprot-Staccato software) [36]. Helices, strands, and loops are, respectively, violet, yellow, and cyan. Ultra-conserved sequences involved in nucleotide binding (G boxes) are delimited by black boxes. Black numbers on the left side of the alignment refer to the sequential numbering, whereas black numbers above the sequences indicate the beginning of a secondary structure/G box motif. The fully conserved residues in such boxes are red and marked by an asterisk. In order to facilitate trans-family comparisons of the MD simulation outputs, an arbitrary numbering was set characterized by the label of the secondary structure segment followed by the amino acid position in that segment. In those cases where the G-boxes overlap with the secondary structure segment, positions refer to the G-boxes.