Figure. 3.
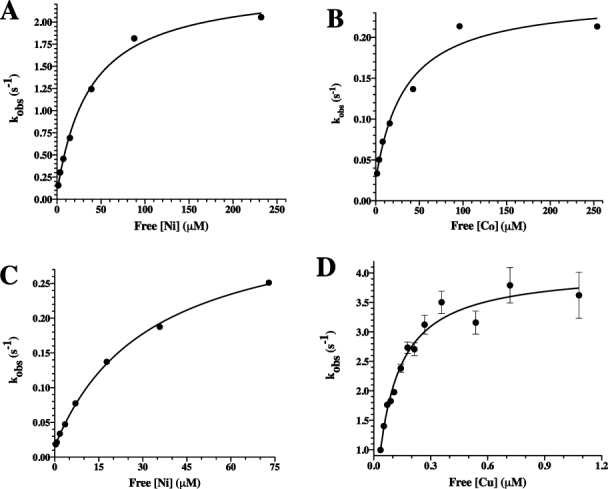
The rate constant, kobs,1, resulting from the approach-to-equilibrium binding studies of Type II metals to PAI-1, shows a hyperbolic dependence on metal concentration. PAI-1 was mixed 1:1 with increasing concentrations of nickel, cobalt, or copper. For nickel and cobalt experiments, data were collected for 500 s and were fit to a triple exponential equation after subtraction of background fluorescence changes, as described in the Materials and Methods section. For copper, the time window was reduced to 50 s, and the data were fit to a double exponential equation after subtraction of background (see Materials and Methods). Changes in the magnitude of kobs,1 versus free metal concentration were plotted for nickel (A) and cobalt (B) in MOPS buffer as well as nickel (C) and copper (D) in Tris buffer. kobs,1 increased in a hyperbolic fashion consistent with a two-step binding model in which there is an initial fast equilibrium binding step followed by a slower conformational change (Scheme 1). Nonlinear least-squares fits to Eq. (1) are shown.
