Figure. 3.
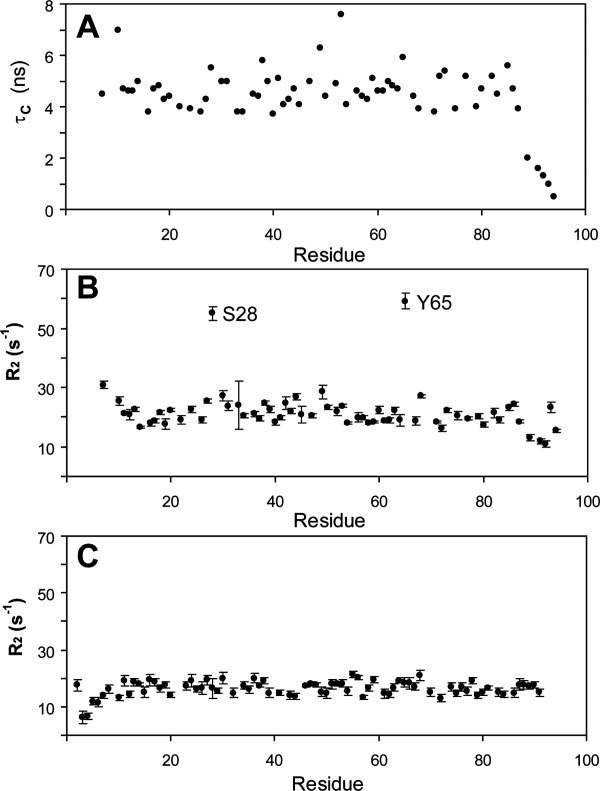
NMR spin relaxation measurements identify dynamic regions of Domain IV that are not present in Domain I. Panel A: A measurement of the rotational correlation time of each NH moiety of Domain IV showed a relatively homogenous distribution with an average for ordered residues of 4.7 ns, suggesting ps-ns timescale motions of the backbone are uniform excepting the highly mobile C-terminal residues. Panel B: Measurements of the R2 spin relaxation rate identified three regions of Domain IV with significantly larger values, suggesting the presence slow μs-ms timescale motions. These residues likewise have greatly reduced or absent intensity in three-dimensional heteronuclear backbone assignment experiments when compared to other residues in the protein. Panel C: Similar transverse relaxation measurements of Domain I (shown) and analysis of triple-resonance experiments identified no regions with enhanced relaxation rates.
