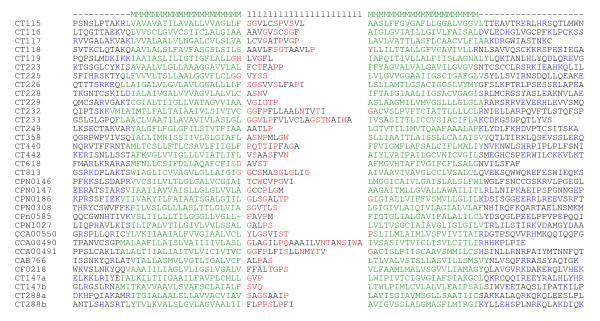
Figure 1.
Composition of the bilobal hydrophobic domain of known Inc proteins. Bilobal domains were aligned manualy utilizing the topological information obtained from Topcons. Identifiers with a and b correspond to the first bilobal (a) and second bilobal (b) domains of CT147 and CT288. Transmembrane residues are in green, flanking regions charged residues in blue, residues with a high potential to form turns in red (turn propensity scale: P>N>R>D>Q>H>K>E>G>W>S>Y>T|C M I V A F L[30]). M = transmembrane domain, l = loop domain. Note that transmembre domain limits were predicted by Topcons and may vary from the limits predicted by Polyphobius and reported in Tables 2, 3 and in the Additional files 1, 2, 3, 4, 5.