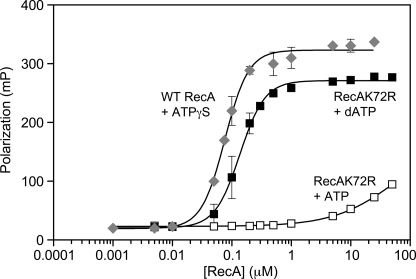
FIGURE 2.
RecA K72R has a defect in ssDNA binding as measured by FP. Direct binding to a fluorescein-labeled 50-nt oligonucleotide by wild type RecA protein in the presence of ATPγS (diamonds) or RecA K72R in the presence of ATP (open squares) or dATP (filled squares) was measured via FP as described under “Experimental Procedures.” The reactions contained 10 nm oligonucleotide, 3 mm ATP, dATP, or ATPγS, and 0.005–50 μm RecA K72R or 0.001–25 μm wild type RecA. Polarization (mP) is plotted versus log scale RecA concentration (μm). Data points represent the mean of three independent trials, and error bars represent 1 S.D. from the mean. The data are fit to a modified form of the Hill Equation as described under “Experimental Procedures.”