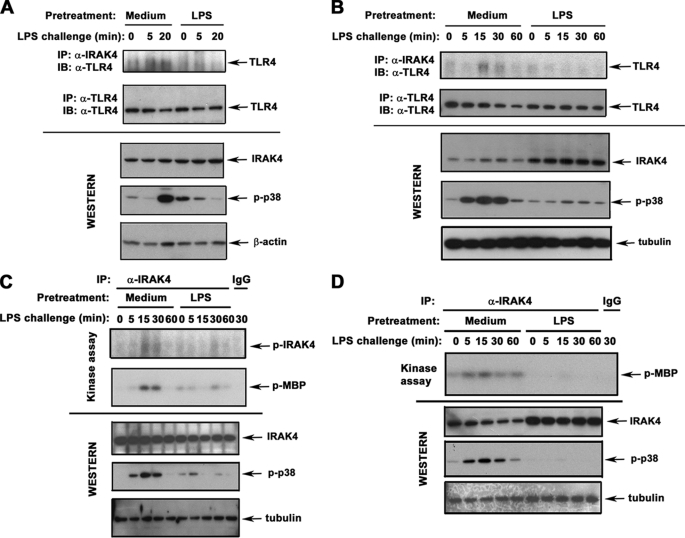
FIGURE 1.
Endotoxin tolerization inhibits LPS-induced IRAK4 recruitment to TLR4 and IRAK4 activation. THP1 cells (A and C) and human monocytes (B and D) were pretreated for 20 h with medium or 10 ng/ml LPS. After washing, the cells were treated for the indicated time points with medium or 100 ng/ml LPS (LPS challenge). A and B, IRAK4 and TLR4 proteins were immunoprecipitated (IP) from whole cell lysates and subjected to immunoblot (IB) analyses with anti-TLR4 Ab, and total expression of IRAK4 proteins was examined by immunoblotting of cell lysates with α-IRAK4 Ab. To assess LPS-inducible cell activation, cell lysates were immunoblotted with anti-p-p38 Ab. β-Actin and tubulin immunoblots were used to control for protein loading. C and D, IRAK4 proteins were immunoprecipitated from cell lysates and subjected to in vitro kinase assays in the absence (IRAK4 autophosphorylation, top panels) or in the presence of MBP (IRAK4 kinase activity, middle panels). Whole cell lysates were immunoblotted with Abs against IRAK4, p-p38, and tubulin to determine total expression levels of IRAK4, LPS inducibility/tolerization, and protein loading, respectively. Shown are the results of a representative (n = 3) experiment.