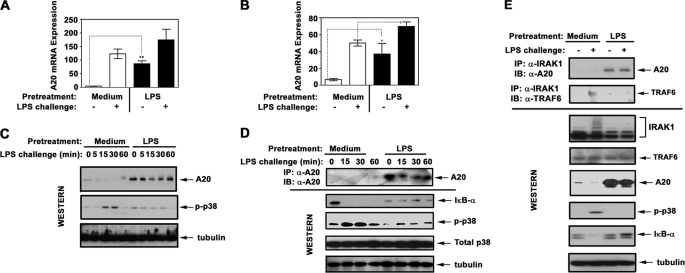
FIGURE 6.
Induction of endotoxin tolerance increases A20 expression and shifts IRAK1 signalosome composition toward accumulation of IRAK1-A20 complexes. THP1 cells (A, C, and E) and human monocytes (B and D) were pretreated for 20 h with medium or 10 ng/ml LPS. Thereafter, the cells were washed and treated for the indicated time periods (A–D) or for 30 min (E) with medium or 100 ng/ml LPS. A and B, RNA was isolated, converted into cDNA, and analyzed by real time PCR with primers specific for hypoxanthine phosphoribosyltransferase and A20. C, A20 proteins were detected by immunoblot (IB) analyses of THP1 whole cell lysates with α-A20 Ab. D, A20 proteins were immunoprecipitated (IP) from whole cell lysates of monocytes with α-A20 Ab and subjected to Western blot analysis using α-A20 Ab. Whole cell lysates were fractionated by SDS-PAGE, electrotransferred onto PVDF membranes, and immunoblotted with Abs against p-p38 (to assess LPS-mediated cell activation/tolerization), total p38, and tubulin (to control for protein loading). The data of a representative experiment (n = 5) are depicted. E, IRAK1 proteins were immunoprecipitated from whole cell lysates and immunoblotted with Abs against A20 and TRAF6. Whole cell lysates were subjected to Western blot analysis with Abs against A20, TRAF6, and IRAK1 to determine their total levels. p-p38 IκB-α immunoblots were performed to examine cell activation/tolerance, and tubulin immunoblots were carried out to control protein loading. The results of a representative experiment are shown.