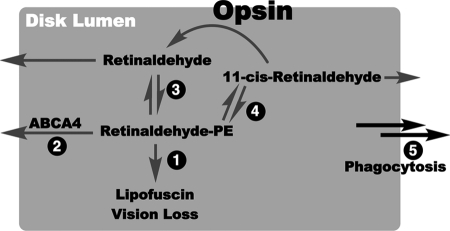
FIGURE 7.
Hypothesized model of lipofuscin prevention by C20-D3-vitamin A. Pathways for retinaldehyde/phosphatidylethanolamine Schiff base (Retinaldehyde-PE) clearance from the disk lumen. The shaded area represents the disk lumen. Clearance pathways are marked 1–5. Arrows going outside the gray area represent movement out of the disk lumen. In theory, the retinaldehyde-PE Schiff base that forms in the rod outer segment after photon isomerization may follow several pathways: 1) it can react to form A2E leading to lipofuscin; 2) it may be actively (ABCA4 mediated) or passively (diffusion) transported out of the disk lumen; 3) it may dissociate back to retinaldehyde and PE and retinaldehyde may then be actively or passively transported out of the disk lumen (79); 4) it may isomerize, dissociate and the generated 11-cis-retinaldehyde may recombine with opsin to form rhodopsin (66) or 11-cis-retinaldehyde and its other isomers may be actively or passively transported out of the disk lumen; and lastly 5) it may be delivered to the RPE directly when the outer segment is phagocytosed. In pathways 2–5 retinaldehyde can presumably be reincorporated back into the visual cycle. Once outside the disk lumen retinaldehyde can be reduced to retinol. In the case of ABCA4 defects leading to Stargardt disease pathway two is slowed resulting in, presumably, an increase in pathway one leading initially to vitamin A dimerization, lipofuscin formation and ultimately vision loss. By the incorporation of deuterium atoms at the C20 position of vitamin A, we have shown in prior work that pathway one becomes higher in energy, and as a result is slowed down. This should allow more time for retinaldehyde to be re-incorporated into the visual cycle via pathways 2–5. Through this mechanism, the use of C20-deuterated vitamin A may be an effective methodology toward mitigating the clinical consequences of alterations in the ABCA4 gene.