FIGURE 5.
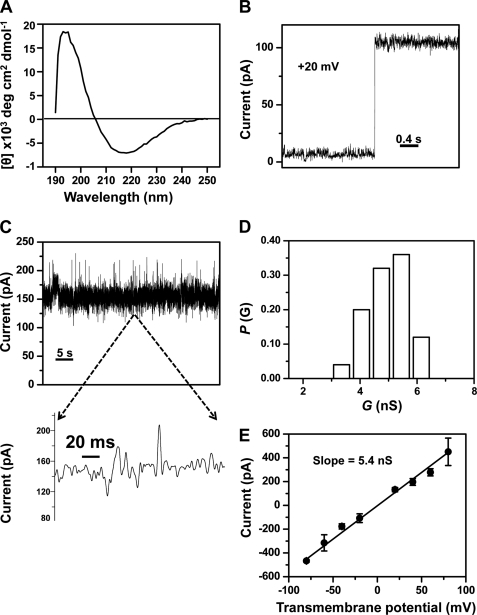
Single-channel electrical recordings of the refolded FhuAΔC/Δ4L (rFhuAΔC/Δ4L) protein. A, circular dichroism spectrum of the rFhuAΔC/Δ4L protein in DDM. 3.42 μm rFhuAΔC/Δ4L protein (see under “Experimental Procedures”) was dialyzed against 5 mm Tris, pH 8.32, 100 mm NaCl, and 0.25% (w/v) DDM, and the measurements were carried out at 20 °C. B, step increase of the electrical current showing a single-channel insertion of the rFhuAΔC/Δ4L into the lipid bilayer. The rFhuAΔC/Δ4L protein was added to the cis side. The transmembrane potential was +20 mV. The increase of current gives a conductance of ∼5 nS. C, single-channel electrical trace of rFhuAΔC/Δ4L at an applied potential of +40 mV. The expanded trace illustrates the signature of the channel at a greater time resolution. D, histogram of the probability (P(G)) of the occurrence of a given single-channel conductance of rFhuAΔC/Δ4L. E, current-voltage relationship of a single rFhuAΔC/Δ4L protein pore. The standard error bars were calculated from at least three separate single-channel experiments. Single-channel electrical recordings were acquired in 1 m KCl, 10 mm potassium phosphate, pH 7.4. The single-channel electrical traces were low pass Bessel-filtered at 2 kHz.