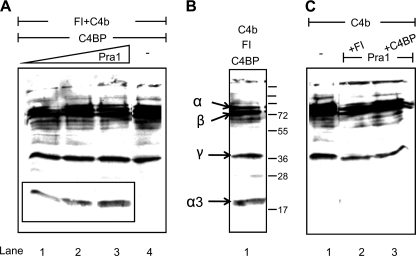
FIGURE 6.
C4BP bound to Pra1 displays cofactor activity. A, Pra1 was immobilized at a concentration of 0.5 (lane 1), 1.0 (lane 2), or 2.0 μg (lane 3), and C4BP was added. After blocking and intensive washing, purified Factor I and C4b were added. Following incubation, the reaction mixture was separated by SDS-PAGE and transferred to a membrane, and C4b as well as C4b cleavage fragments were identified by Western blotting using polyclonal goat C4 antiserum. Cofactor activity of C4BP bound to Pra1 is indicated by the appearance of the 20-kDa α3-fragment (A, lanes 1–3). No cleavage products were identified when Pra1 was absent (A, lane 4). C4BP incubated with Factor I and C4b was used as a positive control to identify C4b cleavage fragments (B). No cleavage occurred in the absence of C4BP or Factor I (C, lanes 2 and 3) or C4b alone (C, lane 1). A representative result is shown of three independent experiments.