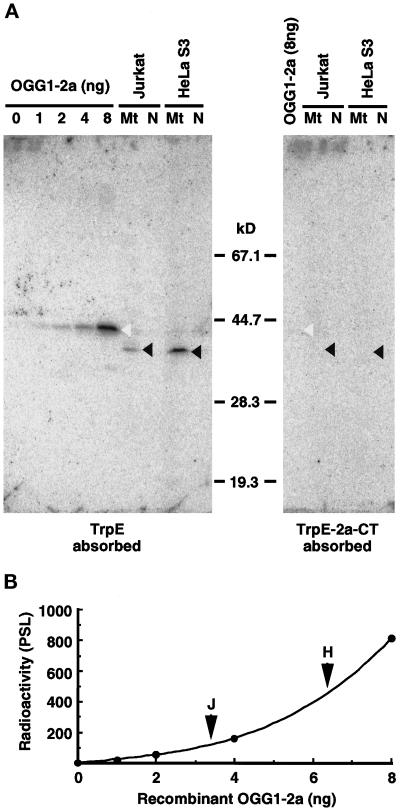
Figure 3.
Detection of the authentic OGG1 protein in human cell lines by Western blotting. (A) Western blotting. Various amounts of the recombinant OGG1–2a expressed in E. coli and isolated mitochondria (Mt) and nuclei (N) (equivalent to 100 μg of protein) from Jurkat and HeLa S3 cells were subjected to Western blot analysis using anti–2a-CT, which was preabsorbed by incubation with an immobilized nonspecific antigen (TrpE) or specific antigen (TrpE-2a-CT), respectively. Blots were probed with 125I-labeled protein A to detect bound antibody and scanned with a Bio-Image analyzer, BAS2000. Open arrowheads indicate bands corresponding to p43, and closed arrowheads indicate bands corresponding to p40, detected by the antibodies preabsorbed with TrpE. (B) Quantitative Western blot analysis of OGG1–2a. Correlation between the amounts of the recombinant OGG1–2a and the radioactivity measured was shown in the plot. Arrowheads indicate radioactivity for the band of p40 detected in each mitochondrial fraction (100 μg of protein) from Jurkat and HeLa S3 cells, respectively.