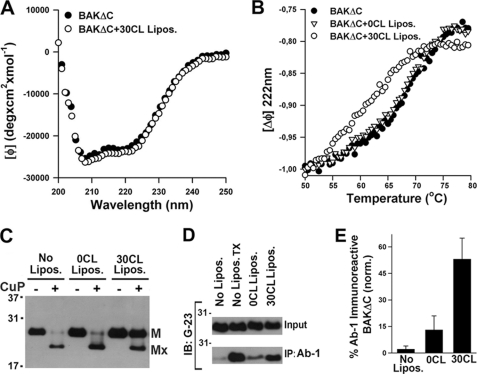
FIGURE 4.
Interaction with CL destabilizes BAKΔC solution fold and exposes amino-terminal regions of the protein. A, the CD spectrum of BAKΔC in the absence (BAKΔC) or presence of LUV composed of 25PC/35PE/10PI/30CL (BAKΔC+30CL Lipos.) is shown. B, thermal denaturation curves for BAKΔC incubated in the absence of LUV (BAKΔC) or in the presence of LUV composed of 55PC/35PE/10PI (BAKΔC+0CL Lipos.) or 25PC/35PE/10PI/30CL (BAKΔC+30CL Lipos.) is shown. Thermal denaturation curves were obtained by recording the temperature dependence of BAKΔC ellipticity at 222 nm. The molar ellipticity at 50 °C was set to −1.0 to compare BAKΔC thermal denaturation rates in the absence and presence of vesicles. BAKΔC and LUV concentrations were 5 μm and 2 mm, respectively. C, BAKΔC (200 nm) was incubated in the absence of liposomes (No Lipos.) or with LUV of the indicated compositions (0CL Lipos. and 30CL Lipos.) followed by treatment with CuPhe or with DMSO vehicle, SDS/PAGE under non-reducing conditions, and immunoblot analysis of BAKΔC content using anti-BAK G23 polyclonal antibody. The positions of monomeric BAKΔC (M) and monomeric BAKΔC harboring an intramolecular cross-link (Mx) are indicated. D, shown is a Western blot of BAKΔC immunoprecipitates (IP) after incubating the protein with 2% CHAPS (No Lipos., negative control) or with 0.2% TX-100 (No Lipos.TX, positive control) or with LUV of the indicated lipid compositions plus 2% CHAPS. Samples were subjected to immunoprecipitation with conformation-specific anti-BAK Ab-1 antibody conjugated to protein A-agarose beads followed by SDS-PAGE/immunoblot (IB) analysis using anti-BAK G23 antibody. Input represents an equivalent amount of BAKΔC loaded in each sample. Concentrations of BAKΔC and LUV were 400 nm and 1 mm, respectively. E, quantitation of BAKΔC immunoreactivity to Ab-1 was assessed by immunoprecipitation and densitometric analysis of immunoblots obtained from three independent experiments as described in panel D.