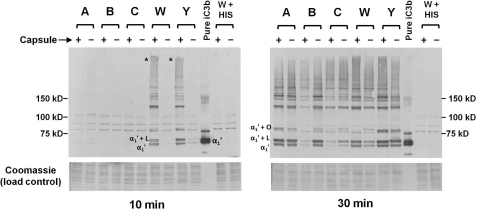
FIGURE 3.
iC3b deposition on isogenic Cap+ and Cap− meningococci. The five isogenic Cap+ and Cap− pairs of N. meningitidis were incubated with C2-depleted human serum for either 10 or 30 min and bacteria-bound iC3b was analyzed by Western blotting with anti-iC3b mAb G-3E. The position of the α1′ (68 kDa) fragment of C3 is indicated in the control lane that contains purified iC3b (see text for an explanation of the α1′ “doublet”). Both blots (10 min (left) and 30 min (right) incubations) were exposed to the alkaline phosphatase substrate for the same duration. “α1′ + L” and “α1′ + O” indicate the positions of adducts of LOS or opacity-associated protein covalently linked to the α1′ iC3b fragment, respectively (36, 46, 47). The asterisks in the “10-min blot” indicate high-molecular mass complexes that contain iC3b in the lanes containing Cap+ W and Y strains. Proteins on the membrane migrating below ∼40 kDa were stained with Coomassie Blue and served as a loading control. Cap+ and Cap− derivatives of strain W171 were incubated with heat-inactivated C2-depleted serum (lanes marked W + HIS) and served as controls to validate the specificity of covalently bound C3 fragments to bacteria.