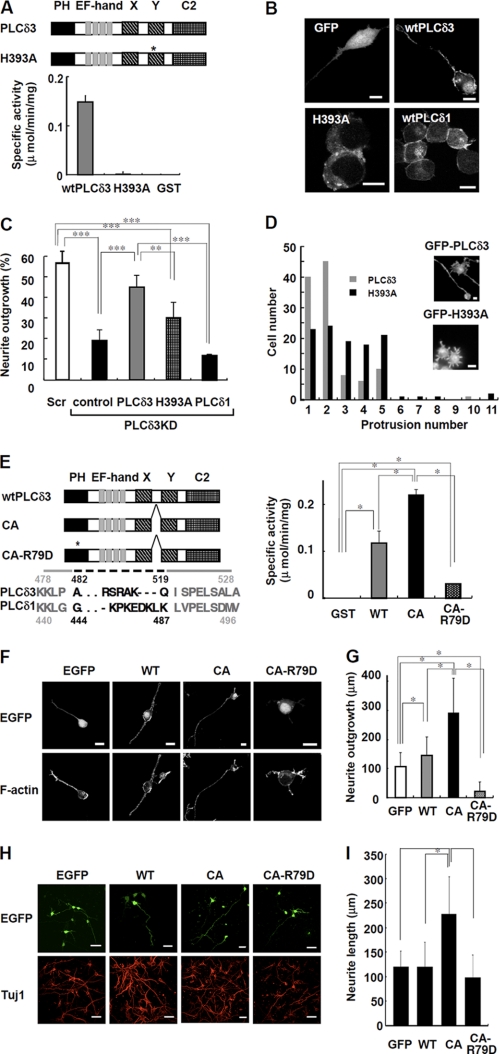
FIGURE 5.
PI(4,5)P2 hydrolyzing activity regulates the neurite numbers and neurite outgrowth upon serum withdrawal. A, PI(4,5)P2 hydrolyzing activity of GST-tagged wild-type PLCδ3 (wtPLCδ3), H393A, or GST. Domain structure of wild-type PLCδ3 and H393A is shown. The asterisk indicates the position of the point mutation in catalytic domain of H393A. Recombinant H393A lacks PLC activity. B, GFP-tagged PLCδ1, PLCδ3, or H393A mutant was transfected into Neuro2a cells for 24 h, and the cells were cultured in DMEM without serum for 48 h. GFP was used as a control. Scale bars, 10 μm. C, effect of catalytic activity on neurite outgrowth. Cells were subjected to PLCδ3 knockdown and subsequently transfected with GFP, GFP-PLCδ3, GFP-H393A, or GFP-PLCδ1. After transfection, the cells were incubated in DMEM without serum for 48 h, and the percentage of neurite outgrowth in GFP-positive cells was quantified and represented in a bar graph (%). Data represent the mean ± S.D. of three experiments. Tukey's test showed significant difference between indicated two groups (**, p < 0.01; ***, p < 0.001). D, cells transfected with mutant H393A show formation of supernumerary protrusions. GFP-PLCδ3 or GFP-H393A was introduced into PLCδ3KD cells, and the cells were stained with rhodamine/phalloidin after a 48-h incubation in DMEM without serum. A histogram displaying the number of cells plotted against the indicated number of neurites/protrusions is shown. The population of GFP-PLCδ3 or GFP-H393A transfected cells showing each neurite/protrusion numbers was analyzed 48 h after serum withdrawal (gray bars, wild-type PLCδ3; black bars, H393A) irrespective of their length. Scale bar, 10 μm. E, PI(4,5)P2 hydrolyzing activity in recombinant GST-tagged PLCδ3, CA, or CA-R79D. Domain structure of wtPLCδ3, CA mutant or its PH domain mutant (CA-R79D) is shown. The asterisk indicates the position of the point mutation of Arg79 in PH domain required for the specific PI(4,5)P2 binding. Dashed line indicates the deleted amino acid residues located between catalytic X and Y region in CA mutant. CA mutant shows higher PLC activity than wtPLCδ3, whereas CA-R79D shows a little activity. Tukey's test showed significant difference between indicated two groups (*, p < 0.05). F, Neuro2a cell expressing CA promotes neurite extension. GFP-tagged wtPLCδ3, CA, or CA-R79D was transfected into Neuro2a cells for 24 h, and the neurite extension was analyzed upon serum withdrawal. CA mutant promoted neurite extension. The point mutation in PH domain abolished the targeting of CA mutant to plasma membrane. Scale bars, 20 μm. G, effect of wtPLCδ3, CA, or CA-R79D on neurite growth was analyzed by quantification of the longest neurite extending from each transfected cell in the absence of serum. The average length was represented as bar graphs (n = 100), and it is notable that CA mutant but not CA-R79D promotes significant neurite outgrowth. The Steel-Dwass multiple comparison test showed significant differences between two conditions (*, p < 0.05). H, constitutively active PLCδ3 effectively promote the neurite outgrowth in cortical neurons. The effect of wtPLCδ3, CA, or CA-R79D tagged with EGFP on neurite growth was analyzed by quantification of the longest neurite from each EGFP-positive cortical neuron (DIV3). The neurons were stained with Tuj1 as a neuronal marker. Scale bars, 40 μm. I, data represent the average neurite length of each transfected neuron (n = 100). The Steel-Dwass test showed significant differences between two conditions (*, p < 0.05).