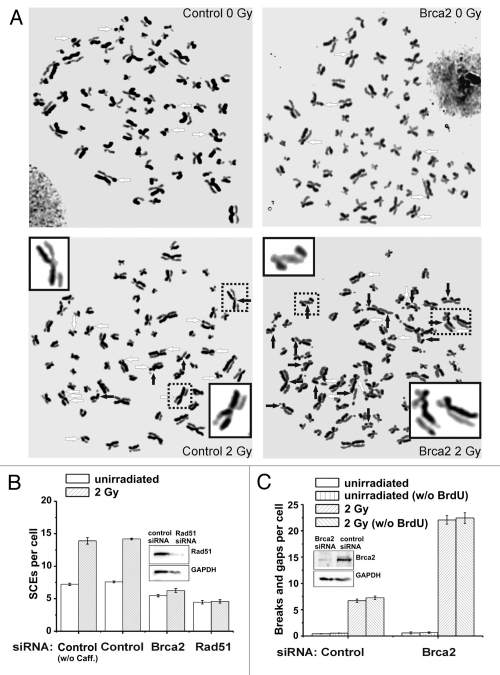
Figure 2.
(A) Representative images of metaphase spreads after standard fluorescence plus Giemsa (FPG) staining. HeLa S3 cells were grown for 48 h (about two rounds of replication) in medium containing 10 µM BrdU. Cells were then irradiated with 2 Gy and aphidicolin (3 µg/ml) was added immediately. 1 mM caffeine (to abrogate the G2 checkpoint) and 200 ng/ml colcemid (to arrest cells in mitosis) were added 6 h later and metaphases were harvested at 9 h after IR. The mean number of chromosomes in HeLa S3 cells varied between 68 and 72, and all data were normalized to 70 chromosomes per cell. Untreated (left parts) and Brca2-siRNA-treated cells are shown (right parts); SCEs are marked by white arrows, chromatid breaks/gaps by black arrows. The 4 insets show examples of a chromosome with a chromatid break/gap (the two upper insets), an SCE (the lower inset on the left) or a chromatid break/gap together with an SCE (the chromosome in the lower inset on the right). (B) SCE analysis of control, Brca2- and Rad51-siRNA-treated HeLa S3 cells. siRNA knock-down of Brca2 and Rad51 results in a complete abolishment of IR-induced SCE formation compared to control cells. Caffeine added at 6 h post IR does not affect the SCE levels. (C) Analysis of chromatid breaks and gaps in control and Brca2-siRNA-treated HeLa S3 cells. Brca2-downregulated cells exhibit an elevated level of chromatid breaks and gaps compared to control cells. We have also measured chromatid breaks and gaps in HeLa S3 cells that were not pre-labeled with BrdU and have obtained similar results. Thus, under our experimental conditions, the radiosensitizing agent BrdU does not significantly affect the breakage level in G2-irradiated cells.41