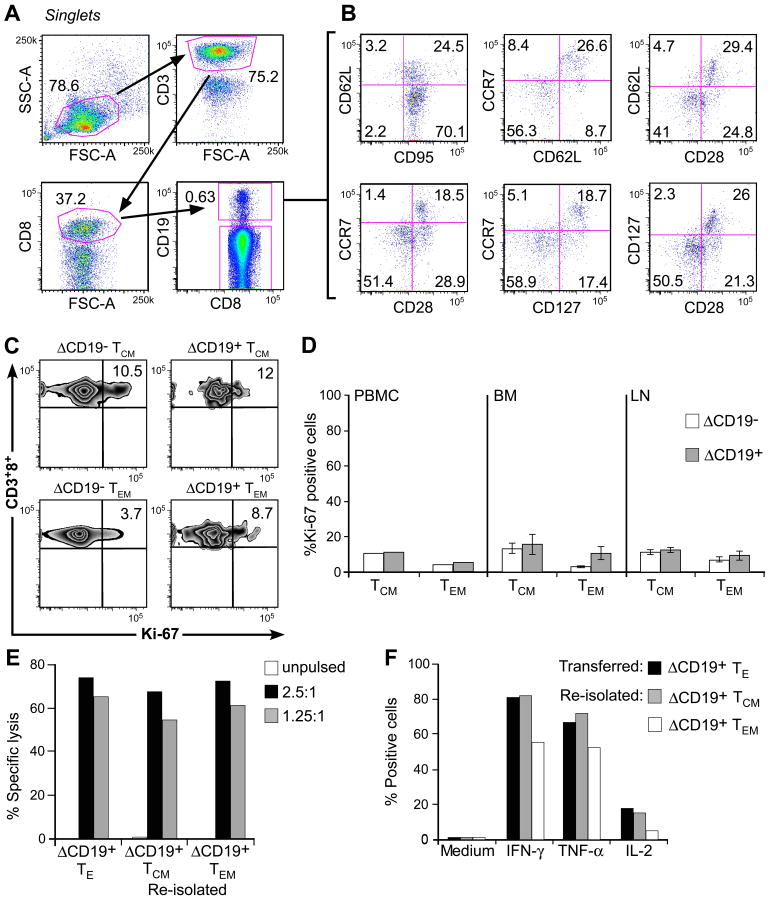
Fig. 6. Analysis of phenotype and function of adoptively transferred ΔCD19+ TCM-derived TE clones.
(A) Multiparameter flow cytometry of macaque PBMC obtained 4 weeks after a ΔCD19+ TE infusion. Transferred ΔCD19+ T cells were identified in PBMC after staining with mAbs to CD3, CD8, and CD19. (B) The expression of TM marker on CD3+CD8+ΔCD19+ T cells was determined by flow cytometry after co-staining with CD62L, CCR7, CD28, CD127 or CD95. (C) Aliquots of macaque PBMC were obtained 8 weeks after infusion of a TCM-derived ΔCD19+CD8+ TE clone. CCR7+CD95+ TCM and CCR7−CD95+ TEM subsets either in the CD3+CD8+ΔCD19− or CD3+CD8+ΔCD19+ cells were identified as described in (A) and (B). Cells were then stained for intracellular expression of Ki-67. Inset values show the frequency (%) of Ki-67+ T cells. (D) Analysis of intracellular Ki-67 in PBMC obtained from 2 macaques 6-8 weeks after infusion of a TCM-derived ΔCD19+CD8+ TE clone, and in samples of BM and LN obtained from 3 macaques 2 weeks after the infusion. PBMC: mean; BM and LN: mean ± SD. (E) Function of re-isolated ΔCD19+ TE obtained from TCM or TEM subsets. Aliquots of post-infusion PBMC were sort-purified in CD8+ΔCD19+CD62L+ and CD8+ΔCD19+CD62L− subsets, restimulated in vitro using anti-CD3/CD28 mAbs, and examined in a chromium release assay for recognition of unpulsed (□) or peptide-pulsed target cells. E/T (■) 2.5:1, ( ) 1.25:1. The transferred ΔCD19+ TE clone served as control. (F) Aliquots of the infused ΔCD19+ TE clone (■) and re-isolated ΔCD19+ TE either obtained from TCM (
) 1.25:1. The transferred ΔCD19+ TE clone served as control. (F) Aliquots of the infused ΔCD19+ TE clone (■) and re-isolated ΔCD19+ TE either obtained from TCM ( ) or TEM (□) were stimulated with medium or CMV peptide-pulsed antigen-presenting cells, and examined by CFC for production of IFN-γ, TNF-α, and IL-2 after gating on CD3+CD8+ T cells.
) or TEM (□) were stimulated with medium or CMV peptide-pulsed antigen-presenting cells, and examined by CFC for production of IFN-γ, TNF-α, and IL-2 after gating on CD3+CD8+ T cells.