Abstract
Objectives
This study aimed to evaluate a novel three-dimensional ultrasound (US) guidance system for use in hepatic microwave ablation (MWA).
Methods
An in vitro assessment was performed in which users with different degrees of experience were evaluated for accuracy in targeting phantom lesions embedded in agar using US alone, or US in conjunction with the InVision™ System (IVS). An eight-patient pilot trial of the IVS was then performed in the setting of open hepatic MWA, in which lesions would otherwise have been targeted with conventional US.
Results
In vitro studies demonstrated that the IVS significantly improved targeting accuracy at all levels of operator experience (novice, beginner and expert). In the human trial, a total of 31 tumours were targeted and all lesions were hit in one pass, as assessed by independent US image observations. There were no adverse operative events; however, there was minor line-of-sight interference with the infra-red tracking mechanism when some lesions high on the dome of the liver were targeted.
Conclusions
The IVS significantly increased the accuracy of complex targeting procedures of phantom lesions and enhanced targeting in an eight-patient clinical pilot study. During the accrual phase of this pilot study, the development of improved non-optical tracking hardware obviated the requirement to maintain a direct line of sight. The trial was then halted prematurely in order to focus on the application of the IVS utilizing this non-optical modality.
Keywords: lesion targeting, ultrasound, image guidance
Introduction
Ultrasound (US) is the current modality of choice for intraoperative imaging of the liver and liver tumours, providing surgeons with real-time anatomic images from a dynamic, mobile surgical field.1,2 Intraoperative US is an essential tool for surgeons during laparoscopic and open hepatic resections, as well as in the targeting of liver lesions for biopsy or ablation.3–5 Despite the versatility and real-time information that US provides, the images are provided in a two-dimensional (2-D) format, which requires the surgeon to mentally reconstruct the spatial relations of the target lesion and hepatic anatomy in three dimensions (3-D). In the setting of lesion targeting for tumour ablation, the position of the lesion in relation to both the intra- and extrahepatic anatomy determines the trajectory at which the ablation antenna must be introduced. When this trajectory or angle is ‘out of plane’ with the 2-D US image, the ablation antenna will not be seen until it crosses the US plane and even then it will only appear as a bright dot on the US image. Conversely, it is much easier to visualize the ablation antenna or biopsy needle when performing a task ‘in plane’. The process of mentally reconstructing a 3-D image of a liver lesion and locating the ablation antenna within the 2-D US image represents an extremely difficult and complex task. Even experienced liver surgeons may have difficulty in interpreting US images of the liver and it is not unheard of for surgeons to invite their radiologist colleagues to the operating theatre for an intraoperative US consult.6 Imprecise tumour targeting can increase the probability of inadvertent injury to adjacent structures and incomplete tumour ablation. For these reasons, difficulties associated with liver tumour targeting during ablation contributed to the significantly high local recurrence rates reported in the early ablation literature.7
An ideal targeting system would combine real-time imaging and versatile positioning of US in a 3-D representation of the targeting equipment (either a biopsy needle or ablation antenna) and the relevant hepatic anatomy, thus minimizing the requirement for mental reconstruction. Such targeting systems have been developed with the aim of improving needle biopsy accuracy for superficially accessible lesions in the breast and prostate.8–10 With specific reference to liver tumour ablation, it has been shown that providing the operator with a 3-D US image improves the accuracy of ablation instrument placement.11,12 These studies were performed with US devices capable of reconstructing a 3-D image after the operator has scanned over the target lesion with a 2-D US handpiece. This 3-D technology is an important aid in pre-ablation planning and in confirming the position of the needle after placement, but it is not capable of real-time 3-D reconstruction during actual needle positioning.
The aim of this study was to assess the clinical applicability of a novel US targeting system capable of providing a real-time 3-D reconstruction of the target lesion and ablation instrument.
The ablation modality of choice in clinical practice at this institution is microwave ablation (MWA), which transmits electromagnetic energy at a high frequency (from 300 MHz to 10 GHz) into the surrounding tissue without requiring the point-to-point current necessary in radiofrequency ablation (RFA).13 Within the microwave field, the heat generates results in a homogeneous and uniform zone of coagulative necrosis that is not susceptible to the heat sink effect seen in RFA when performed near blood vessels.14 This results in a more predictable and consistent ablation zone, which is associated with lower recurrence rates when utilized for hepatic tumour ablation.15
Materials and methods
Institutional assurances
Approval was obtained from the Carolinas Medical Center Institutional Review Board for a human pilot study of 10 patients utilizing the InVision™ System (IVS) for guidance in open MWA of liver tumours that would otherwise be targeted with conventional 2-D US. The decision to use MWA as opposed to other modalities was not altered by inclusion in this study. Informed consent was obtained from all patients.
Overview of the targeting system
The InVision™ System (InnerOptic Technology, Inc., Hillsborough, NC, USA) is a computer-based image guidance system. The IVS is linked to a commercially available infra-red tracking system (Northern Digital, Inc., Waterloo, ON, Canada) which monitors the position of specialized reflective ‘tracking mounts’ that are attached to the US handpiece and ablation antenna (Fig. 1). Dual infra-red cameras, attached to a boom and separated by a fixed distance, observe the tracking mounts as they are moved within the operative field. From these two stereo video streams, the position and orientation of the tracking mounts is triangulated continuously. In this aspect, the system is not unlike any other infra-red motion detection system.
Figure 1.
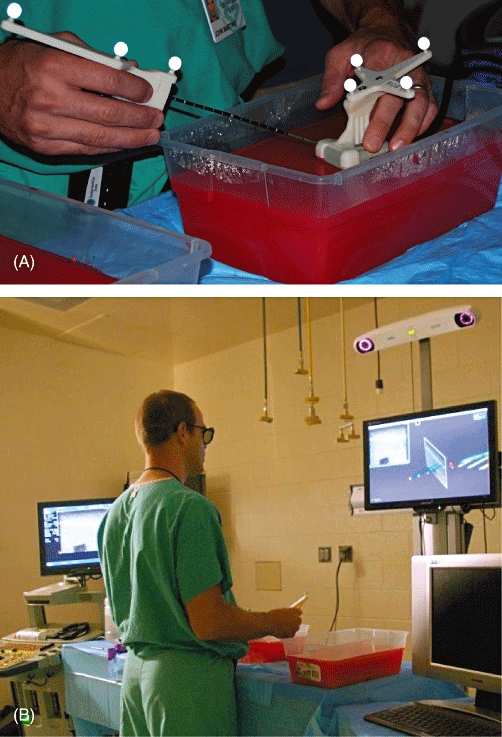
(A) Microwave antenna and ultrasound handpiece fitted with specialized retro-reflectors used with the InVision™ System (IVS). Agar blocks containing phantom tumours were made for ex vivo instrument evaluation. (B) Operative performing ex vivo phantom tumour location and antenna placement using the IVS
The novel aspect of the IVS refers to how the real-time position and orientation of the tracking mounts are acquired from the infra-red cameras and translated into a virtual representation of the ablation antenna and US image in three dimensions on a video monitor.
Before the system is used, a computer-generated virtual representation of the ablation antenna, based on its exact size and shape, is created and stored in the computer. Then, during the targeting procedure, the IVS software acquires information from the infra-red cameras as they follow the position and orientation of the tracking mount on the real antenna. The software then combines this positional information with the stored virtual representation of the antenna. This combination of real-time positional information and virtual antenna image is then displayed on a 3-D LCD monitor (Miracube/Pavonine Korea, Inc., Incheon, South Korea). Thus, the virtual antenna image on the monitor screen moves in three dimensions and in real time as the operator moves the actual antenna in the surgical field.
In detail, the actual flat 2-D US image is displayed on the 3-D monitor so that the operator is able to observe the target lesion and surrounding anatomy in real time, as with any conventional 2-D US imaging system. At the same time, however, the infra-red cameras track the position and orientation of the tracking mount on the US handpiece and feed this information to the IVS software in real time. The IVS software then links this positional information to the 2-D US image. Thus, as the operator rotates or moves the US handpiece, the flat 2-D US image on the monitor rotates and moves in three dimensions.
The operator then puts on passive stereo glasses (similar to those used in 3-D movie theatres). Now the operator sees, on a 3-D monitor, a flat 2-D US image and a virtual representation of the ablation antenna moving in exact relation to one another and exactly mimicking the orientation and movement of the real US handpiece and ablation antenna in the operative field (Fig. 2). When used clinically for ablation, the IVS software also provides a 3-D representation of the predicted ablation zone in order to facilitate visual confirmation that the intended target is within range for complete ablation (Fig. 2C, D).
Figure 2.
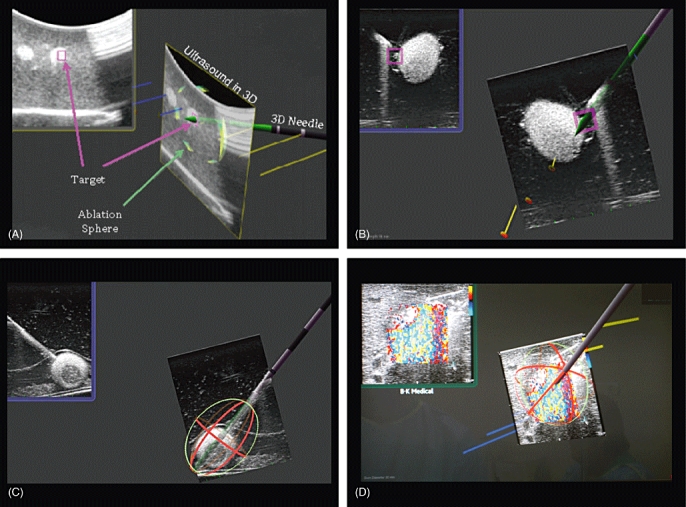
Representative screen shots taken from the monitor demonstrating in 3-D the 2-D ultrasound (US) image, virtual ablation antenna and predicted antenna tract. (A, B) Representative screen shots of tumour targeting. (C) Representative screen shot of a phantom tumour in an experimental pig model showing the 3-D representation of the predicted ablation zone. (D) Photograph of monitor displaying imaging performed during microwave ablation (MWA) of a hepatic tumour in the human MWA study, showing 3-D representation of the predicted ablation zone
The computational core of the system consists of proprietary software developed by InnerOptic Technology, Inc., which is run on a standard, commercially available desktop computer (Dell Optiplex 755; Dell, Inc., Hillsboro, OR, USA) using a high-performance graphics card (GeForce 9400GT; Nvidia Corp., Santa Clara, CA, USA).
Preclinical experimental design
To evaluate the targeting system for use in MWA, a phantom tumour model was developed to compare the accuracy of antenna placement between conventional 2-D US and the novel guidance system. Phantom tumours were created by embedding 6–8-mm fragments of egg white approximately 3 cm deep into an opaque agar gel block (Fig. 1A). A B-K Pro Focus 2202 US device with a surgical T-probe (B-K Medical A/S, Herlev, Denmark) was used alone for conventional US or in conjunction with the IVS (Fig. 1). A standard 13-gauge microwave antenna (Valleylab, Inc., Boulder, CO, USA) was used for all targeting attempts. Three operators with varying degrees of experience in US were compared. The operators included a fourth-year medical student with no US or targeting experience (novice), a research fellow who had completed general surgery training and had some US targeting experience (intermediate), and a hepatopancreatobiliary (HPB) attending doctor with extensive US guided targeting experience (expert).
The ablation antenna was placed using standard procedures for liver tumour MWA. The phantom was first located with the US handpiece and the antenna introduced at one of three designated angles (0°, 45°, 90°) relative to the US plane (0° corresponded to ‘in plane’ and 90° corresponded to ‘out of plane’). For each attempt, the angle was chosen randomly. Each user made 10 needle placement attempts at each of the three angles, with and without the guidance system. Use of the guidance system vs. conventional US was randomized across placement attempts. Users were not allowed to alter the course of the needle antenna once the agar block had been punctured. Data on the accuracy of antenna placement were collected by US confirmation in two orthogonal planes with the antenna in the final position selected by the operator. For the attempt to qualify as a ‘hit’, the tip was required to penetrate the target; however, if the needle tip passed through the target entirely, the attempt was recorded as a ‘miss’.
Human pilot trial design
A human pilot study with eight patients was performed utilizing the IVS for open MWA of liver tumours that would otherwise be targeted with conventional 2-D US. Following the administration of general anaesthesia, the abdomen was prepped and draped in the usual fashion and the infra-red tracking system and monitor of the guidance system were positioned at the head of the patient (Fig. 3A). The reflective tracking mounts were fitted to a B-K Pro Focus 2202 US device with a surgical T-probe and one of two different MWA antennae in a sterile fashion using conventional US sleeves (Fig. 3B). Both commercially available MWA systems were employed: one is a 915-MHz system (Valleylab, Inc.) and the other is a 2.45-GHz system (Acculis Ltd/Microsulis Medical Ltd, Denmead, UK). In each patient, the surgeon (JBM, DAI) chose the particular system and antenna based on the characteristics of each patient's tumours. The open MWA proceeded in the usual fashion through an upper midline or subcostal incision and retractors were placed as needed. Ultrasound images from each targeting attempt were recorded for independent review by a radiologist experienced in percutaneous tumour ablation. The number of tumours targeted, tumour size, MWA system used, number of passes required to hit the lesion, percentage of lesions hit and adverse events were recorded. Stored US images were evaluated by an independent radiologist to confirm the accuracy of targeting.
Figure 3.
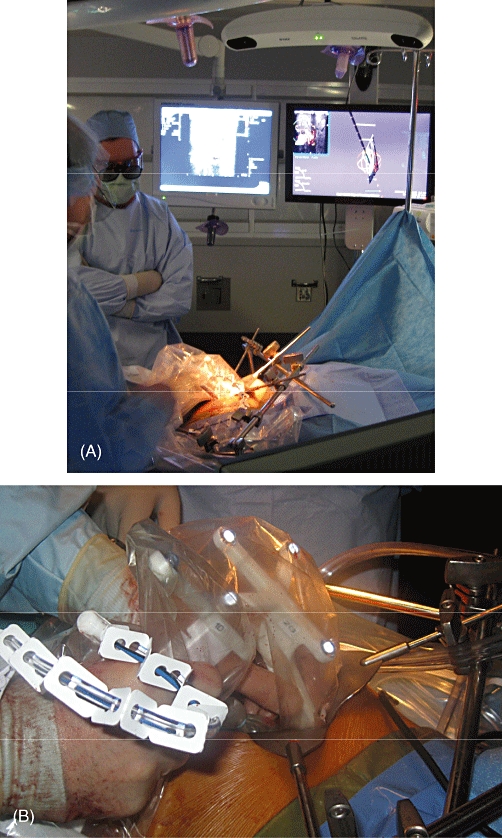
(A) Operating room positioning of the infra-red tracking system and monitors. (B) Reflective tracking mounts fitted to a B-K ProFocus 2202 ultrasound (US) device and a microwave ablation (MWA) probe wrapped with US sleeves as used to perform an open MWA of a human hepatic lesion
Statistical analysis
Results are expressed as simple counts. Groups were compared using chi-squared analysis. A P-value of <0.05 was considered statistically significant. jmp Version 8 (SAS Institute, Inc., Cary, NC, USA) was used for statistical analysis.
Results
Preclinical data
Data comparing operator status and the accuracy of needle placement using either 2-D US or the IVS are shown in Table 1. When all operators were assessed together, accuracy was found to improve significantly at all angles with use of the IVS (Table 2). Of further note, the novice using the IVS achieved a higher rate of accuracy than the expert using conventional US (P < 0.05) (Table 1).
Table 1.
Laboratory targeting data using conventional 2-D ultrasound (US) (non-guided) or the InVision™ System
| Hits at 0 degrees, n | Hits at 45 degrees, n | Hits at 90 degrees, n | Total hits (n= 30 attempts) | |
|---|---|---|---|---|
| Novice | ||||
| Non-guided | 3 | 3 | 0 | 6 |
| InVision™ System | 9 | 10 | 6 | 25 |
| Intermediate | ||||
| Non-guided | 4 | 2 | 2 | 8 |
| InVision™ System | 8 | 9 | 7 | 24 |
| Expert | ||||
| Non-guided | 5 | 7 | 6 | 18 |
| InVision™ System | 10 | 10 | 10 | 30 |
n= 10 attempts at antenna placement per group (non-guided vs. InVision™ System) per angle (0°, 45°, 90°), where 0° is parallel to or ‘in plane’ with the US image and 90° is orthogonal to or ‘out of plane’ with the US image
Table 2.
Combined data from all users comparing conventional 2-D ultrasound (US) with the InVision™ System at each angle in respect to the 2-D US plane
| Non-guided (n= 30 attempts) | InVision™ (n= 30 attempts) | P-value | |
|---|---|---|---|
| Hits at 0°, n | 12 | 27 | <0.001 |
| Hits at 45°, n | 12 | 29 | <0.001 |
| Hits at 90°, n | 8 | 23 | <0.001 |
n= 30 attempts at antenna placement per group (non-guided vs. InVision™ System) per angle (0°, 45°, 90°), where 0° is parallel to or ‘in plane’ with the US image and 90° is orthogonal to or ‘out of plane’ with the US image
Human pilot study
Eight patients were enrolled in the study, presenting a total of 31 tumours to be targeted. Median tumour diameter was 1.5 cm (range: 0.4–8.0 cm). All 31 lesions were hit in one pass and accurate antenna placement was confirmed by an experienced independent radiologist who reviewed all US images for each patient. There were no adverse events in the operative or postoperative settings. Although the IVS software and visual display technology greatly simplified the targeting process, it was noted that the application of the infra-red tracking system in the setting of open hepatic tumour ablation resulted in transient line-of-sight issues in three of the eight patients, whereby the abdominal wall retractors or abdominal wall obscured the path from the infra-red camera to the tracking mounts attached to the instruments, thus momentarily interfering with transmission to the IVS software. This disruption had no detrimental effect on the accuracy of lesion targeting, but it made hand positioning and the actual task of ablation difficult at times. The IVS software and visual display technology were subsequently adapted to a non-optical tracking system to eliminate these line-of-sight difficulties during open procedures and to expand the role of this targeting technology to the laparoscopic setting. The decision was then made to halt accrual into this open MWA trial and to continue further clinical investigation with the IVS utilizing the non-optical tracking modality in a laparoscopic setting.
Discussion
Ultrasound guidance is indispensable in the intraoperative targeting of hepatic lesions for biopsy or ablation, and accuracy in this targeting is paramount to avoid injury to adjacent tissue and to deliver adequate thermal energy to the target lesion.5–7 Other forms of imaging, such as computed tomography or magnetic resonance imaging, can provide detailed 3-D models for preoperative planning, yet these modalities lack the ability to give second-to-second real-time feedback to the operator as the diaphragm and liver move with respiration. Only US offers this ability; however, US also has disadvantages that must be addressed. Ultrasound targeting of hepatic lesions is hindered by the complex vascular and biliary anatomy of the liver, as well as by the physical constraints of the abdominal cavity. As a result, the targeted lesion must often be approached ‘out of plane’ to the 2-D US image. This imposes a task of increased mental complexity as the operating surgeon must then ‘recreate’ a 3-D image of the lesion from the information available. With these constraints in mind, an image guidance system that is able to decrease the mental and proprioceptive challenges of hepatic tumour targeting while maintaining the versatility and real-time feedback afforded by US has been developed.
These preclinical data clearly demonstrate that this novel 3-D US guidance system allows even those operators with little or no experience in using US or targeting lesions to accurately target phantom lesions that they would otherwise have missed. In addition, the novice or intermediate operator using this targeting system was more accurate than the experienced HPB attending surgeon using conventional 2-D US. However, the novel targeting system also allowed the expert operator to improve antenna placement significantly. By visually representing the exact relationship between instruments and operator, the system significantly reduces the cognitive load borne by the surgeon and increases the accuracy of targeting.
The results of this human pilot study in applying this guidance system to open MWA revealed that the targeting accuracy seen in the laboratory can be attained in the complex environment of open liver surgery with little technical difficulty. The system was easy to set up and manoeuvre in the operating room and targeting was greatly simplified by the IVS software, even for HPB surgeons experienced in hepatic lesion targeting and ablation. The only drawback of the system was the aforementioned line-of-sight issue that was a product of the infra-red tracking system used. This problem arose when operators targeted lesions high on the dome of the liver for which the required angle of approach, in conjunction with the abdominal wall and retractors, obscured the position of the tracking mounts, thus transiently disabling the infra-red tracking system.
It was promptly recognized that any obscuration of the line of sight would be an occasional minor inconvenience in open ablation, but would represent a major impediment to the development of a laparoscopic version of this technology. As the facility to use this system in minimally invasive procedures would greatly broaden its applicability, the distinct advantages of the IVS software and image display technology were then coupled with a non-optical tracking modality for use in open or laparoscopic procedures. As a single prototype IVS unit had been used in this investigation and the advantages and limitations conferred by the infra-red tracking modality were well delineated after trialling in eight patients, we decided to continue clinical investigation with the IVS and the non-optical tracking modality in a laparoscopic setting.
In summary, this study demonstrates that the incorporation of a real-time 3-D US guidance system significantly increases the accuracy with which complex targeting procedures can be performed. The IVS software and image display technology greatly reduce the mental complexity of lesion targeting during open liver ablation procedures. The only obstacle encountered involved the infra-red tracking system; this will need to be addressed using a non-optical tracking modality that does not depend on the presence of a clear path from the sensor to the instruments, thus allowing this highly applicable technology to move forward to a minimally invasive approach. Current investigations revolve around the incorporation of low-frequency magnetic positioning into the InVision™ System.
Acknowledgments
The authors thank B-K Medical A/S, Herlev, Denmark and InnerOptic Technology, Inc., Hillsborough, NC, USA for equipment support.
Conflicts of interest
Equipment for this study was provided by B-K Medical A/S and InnerOptic Technology, Inc.
References
- 1.Machi J, Oishi AJ, Furumoto NL, Oishi RH. Intraoperative ultrasound. Surg Clin North Am. 2004;84:1085–1111. doi: 10.1016/j.suc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Zacherl J, Scheuba C, Imhof M, Zacherl M, Langle F, Pokieser P, et al. Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg. 2002;26:550–554. doi: 10.1007/s00268-001-0266-2. [DOI] [PubMed] [Google Scholar]
- 3.Torzilli G, Makuuchi M. Intraoperative ultrasonography in liver cancer. Surg Oncol Clin North Am. 2003;12:91–103. doi: 10.1016/s1055-3207(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 4.Cervone A, Sardi A, Conaway GL. Intraoperative ultrasound (IOUS) is essential in the management of metastatic colorectal liver lesions. Am Surg. 2000;66:611–615. [PubMed] [Google Scholar]
- 5.Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi AJ, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumours: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg. 2001;5:477–489. doi: 10.1016/s1091-255x(01)80085-8. [DOI] [PubMed] [Google Scholar]
- 6.Siperstein AE, Rogers SJ, Hansen PD, Gitomirsky A. Laparoscopic thermal ablation of hepatic neuroendocrine tumour metastases. Surgery. 1997;122:1147–1154. doi: 10.1016/s0039-6060(97)90221-x. [DOI] [PubMed] [Google Scholar]
- 7.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surry KJ, Smith WL, Campbell LJ, Mills GR, Downey DB, Fenster A. The development and evaluation of a three-dimensional ultrasound-guided breast biopsy apparatus. Med Image Anal. 2002;6:301–312. doi: 10.1016/s1361-8415(02)00087-7. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal M, State A, Lee J, Hirota G, Ackerman J, Keller K, et al. Augmented reality guidance for needle biopsies: an initial randomized, controlled trial in phantoms. Med Image Anal. 2002;6:313–320. doi: 10.1016/s1361-8415(02)00088-9. [DOI] [PubMed] [Google Scholar]
- 10.Bax J, Cool D, Gardi L, Knight K, Smith D, Montreuil J, et al. Mechanically assisted 3-D ultrasound guided prostate biopsy system. Med Phys. 2008;35:5397–5410. doi: 10.1118/1.3002415. [DOI] [PubMed] [Google Scholar]
- 11.Xu HX, Yin XY, Lu MD, Xie XY, Xu ZF, Liu GJ. Usefulness of three-dimensional sonography in procedures of ablation for liver cancers: initial experience. J Ultrasound Med. 2003;22:1239–1247. doi: 10.7863/jum.2003.22.11.1239. [DOI] [PubMed] [Google Scholar]
- 12.Rose SC, Hassanein TI, Easter DW, Gamagami RA, Bouvet M, Pretorius DH, et al. Value of three-dimensional US for optimizing guidance for ablating focal liver tumours. J Vasc Interv Radiol. 2001;12:507–515. doi: 10.1016/s1051-0443(07)61892-2. [DOI] [PubMed] [Google Scholar]
- 13.Sindram D, Lau KN, Martinie JB, Iannitti DA. Hepatic tumour ablation. Surg Clin North Am. 2010;90:863–876. doi: 10.1016/j.suc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Padma S, Martinie JB, Iannitti DA. Liver tumour ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619–634. doi: 10.1002/jso.21364. [DOI] [PubMed] [Google Scholar]
- 15.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumours: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]


