Abstract
Climate is changing at a fast pace, causing widespread, profound consequences for living organisms. Failure to adjust the timing of life-cycle events to climate may jeopardize populations by causing ecological mismatches to the life cycle of other species and abiotic factors. Population declines of some migratory birds breeding in Europe have been suggested to depend on their inability to adjust migration phenology so as to keep track of advancement of spring events at their breeding grounds. In fact, several migrants have advanced their spring arrival date, but whether such advancement has been sufficient to compensate for temporal shift in spring phenophases or, conversely, birds have become ecologically mismatched, is still an unanswered question, with very few exceptions. We used a novel approach based on accumulated winter and spring temperatures (degree-days) as a proxy for timing of spring biological events to test if the progress of spring at arrival to the breeding areas by 117 European migratory bird species has changed over the past five decades. Migrants, and particularly those wintering in sub-Saharan Africa, now arrive at higher degree-days and may have therefore accumulated a ‘thermal delay’, thus possibly becoming increasingly mismatched to spring phenology. Species with greater ‘thermal delay’ have shown larger population decline, and this evidence was not confounded by concomitant ecological factors or by phylogenetic effects. These findings provide general support to the largely untested hypotheses that migratory birds are becoming ecologically mismatched and that failure to respond to climate change can have severe negative impacts on their populations. The novel approach we adopted can be extended to the analysis of ecological consequences of phenological response to climate change by other taxa.
Keywords: climate change, conservation, ecological mismatch, migration, population trend
1. Introduction
Climatic variation is a major ecological and evolutionary force acting on populations both directly (via its effects on abiotic conditions) and indirectly (through interactions among species within biological communities) [1]. Organisms are selected to track this variation, and failure to adaptively respond to it may cause demographic decline and drive populations to the verge of extinction [2,3].
The Northern Hemisphere has undergone rapid warming during the past decades [4], and there is overwhelming evidence that the phenology and ecology of populations, the distribution of species and the composition of communities show coherent symptoms of climate change effects [5–8]. The increase in winter and spring temperatures at medium and high latitudes [9] has led to a generalized advancement of spring phenological events (e.g. leaf unfolding and flowering) [7,8]. Populations of consumers must track such changes in order to retain an appropriate matching to extrinsic conditions and to the phenology of the organisms to which they are ecologically linked. However, response to climate change may not occur at the same pace among species [7,10,11]. This can be speculated to occur because differences in genetic variance affect the rate of their microevolutionary change, behavioural or physiological traits constrain phenotypic plasticity in phenology, the patterns of climate change in the wintering and passage areas of migratory species differ, or a combination of these conditions (see [12–15]). Phenological shifts in ecological communities may therefore result in directional selection for earlier arrival and breeding [11,16], and those species that lag behind are expected to suffer increasing levels of ‘ecological mismatch’.
Migratory birds breeding in temperate and high-latitude habitats with strong seasonal variation in ecological conditions must optimize the timing of spring arrival [17]. Arriving too early can entail considerable viability costs because of the risk of facing adverse weather or still-poor food supply [17]. On the other hand, late arrival can depress fitness because of reduced success in competition for mates and territories, and mistiming of reproduction with respect to seasonal peaks in food abundance [18]; several studies have indeed shown directional selection for early arrival and breeding of migratory birds [17].
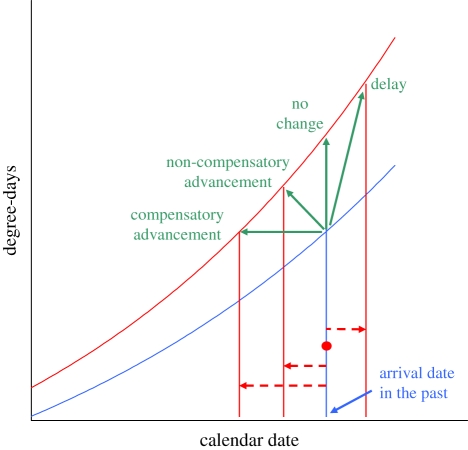
In the Northern Hemisphere, migratory birds have advanced spring arrival to their breeding sites [19,20]. These changes may reflect either microevolution or phenotypically plastic responses to novel climatic conditions [12,13,21,22]. Whether advancement in arrival has allowed birds to fully compensate for advancement of spring events and retain the same level of matching to spring phenology events as in the past or, conversely, only partial compensation has led to increasing levels of ecological mismatch (figure 1), however, remains largely unknown, as evidence exists for very few species and sites [14,23].
Figure 1.
Potential effects of relative climatic and phenological changes on ecological mismatch of migratory birds. Curves represent the progress of spring (degree-days) in 2 years. The curve for the recent year (red line) lies above that for the past (blue line) because of winter and spring warming. Migratory birds show no change (red dot), advancement or delay (red arrows) in arrival date. Species that now arrive at the same or later dates face higher degree-days and are thus ‘thermally delayed’ relative to spring phenophases. Even species that have advanced their arrival may experience a thermal delay, if advancement does not fully compensate for increasing temperatures. Only large advancement in arrival can fully compensate for climate change.
The analysis of the consequences of climate change in terms of mistiming of avian arrival and breeding relative to spring phenological events is hampered by the diversity of the organisms (at different trophic levels) with which birds are ecologically interacting. Moreover, information on the progress of spring events on a daily basis obtained in a methodologically consistent way across decades and diverse geographical regions does not exist for individual plant and invertebrate species, not to mention entire communities. However, at medium and high latitudes, the phenophases and development of plants and ectothermic animals that birds use as, for example, food are strongly dependent on weather, and temperature qualifies as the most important factor [24–29], although other factors (e.g. photoperiod; see [30]) can also intervene. The phenology of several plants and animals integrates the thermal signal over periods spanning weeks to months, so that long-term changes in temperature during the first months of the year will result in a spring phenological response [8]. In addition, temporal shifts in plant phenology generate cascading effects on organisms at higher trophic levels [2,8,31]. The progress of spring at a given date can thus be conveniently expressed by degree-days (DD; the sum of mean daily temperatures above a given threshold from a biologically meaningful start date till the date of interest) indices, which summarize the thermal conditions affecting phenology ([27–29]; see also electronic supplementary material), although, to the best of our knowledge, this approach has never been applied in the studies of avian phenology [32].
By relying on DD indices, we here first provide a direct test of the hypothesis that the progress of spring phenological events at arrival of 117 European migratory bird species to the breeding quarters has changed during recent decades, indicating that birds are experiencing increasing levels of ecological mismatch (see also §3). The analyses are based on a large sample of 242 time series of mean/median spring arrival date (MAD) recorded over 51 years (1958–2008) at four sites in northern Europe (figure 2). Migratory bird species show considerable variation in the timing and advancement of spring arrival, and this variation is partly associated with migration strategy—that is, whether a species is a ‘long-distance’ migrant (LDM) wintering south of the Sahara or it is a ‘short-distance’ migrant (SDM) wintering in Europe or in North Africa ([19,20]; but see [33]). We therefore also tested whether DD values at arrival have changed differently between these two groups of migrants, while predicting that LDMs have suffered larger thermal delay owing to their smaller rate of advancement of spring arrival.
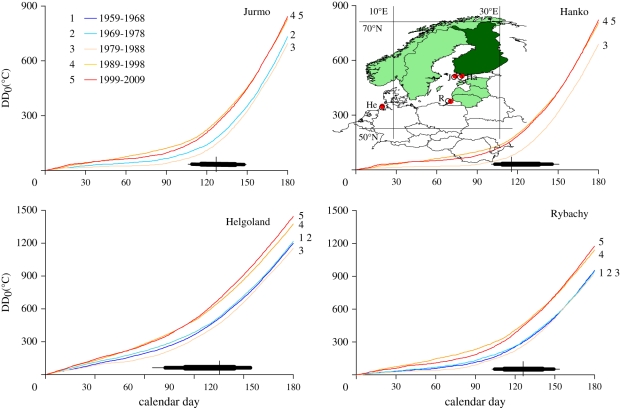
Figure 2.
Temporal variation in winter and spring accumulation of degree-days (DD0). Data refer to the period 1 January–30 June at four study sites and are averaged among years within five periods (1–5). Only periods with avian phenological data at individual sites are presented. Recent periods have markedly higher DD0 values. Inset map shows the study sites (J, Jurmo; Ha, Hanko; He, Helgoland; R, Rybachy), the temperature data grid points (blue dots) and the seven countries relevant to population trend analyses. Trends in Finland were also analysed separately; see text. Box plots indicate the frequency distribution of migration dates: thin boxes: 5–10% or 90–95% of the species; medium-size boxes: 10–25% and 75–90%; thick box: 25–75%; vertical line: median.
Increasing ecological mismatch can cause populations to decline. Under the assumption that higher DD at arrival imply increasing ecological mismatch, we thus also predicted that species with larger increase in DD at arrival showed more negative population trends, and tested this prediction using demographic data for northern Europe [34] as well as for Finland alone, as also obtained from a different source [35]. In the analyses, we controlled for the potentially confounding effects of species ecology, trophic level and migration strategy, as well as for similarity in response owing to common ancestry.
2. Methods
(a). Avian phenological data
Phenological time series (n = 242) were obtained at four sites in northern Europe (Helgoland: 54.18° N, 7.88° E, n = 24; Rybachy: 55.08° N, 20.73° E, n = 32; Jurmo: 59.83° N, 21.62° E, n = 95; Hanko: 59.82° N, 22.90° E, n = 91) for a total of 117 species. The average duration (first to last year) of the time series was 37.0 (6.85 s.d.) years. Data were not available for some species and years, yielding an average number of phenological data points per time series of 34.0 (9.11 s.d.). Their temporal spans (mean year of start to mean year of end across species within sites) were: Helgoland, 1960.1–2007.8; Rybachy, 1959.0–2003.3; Jurmo, 1970.7–2007.8; Hanko, 1979.1–2007.9. Annual arrival dates were expressed as mean (Rybachy and Helgoland) or median (Hanko and Jurmo) date of capture/observation (MAD). Methods of the trapping/observation procedures for estimating MAD are reported in [33,36,37].
(b). Degree-days data
Temperature data were obtained from the ERA-40 and ERA-Interim archives of the European Center for Medium Range Forecasts for the 1.125° latitude × 1.125° longitude grid-point closest to individual sites (see electronic supplementary material). Because temperature thresholds relevant to the phenology of different organisms differ, we considered −3°C, 0°C or +3°C as thresholds, although only the analyses based on DD with the 0°C threshold (DD0) are presented in the main text (see electronic supplementary material for a justification of these thresholds and for analyses based on DD−3 and DD+3). For each year and jth day at each of the four sites, we thus computed degree-days (DDi; i = −3°C, 0°C or +3°C) as the sum of the instantaneous temperatures above −3°C, 0°C or +3°C from 1 January to day j, and divided it by 4 (i.e. the number of instantaneous temperature estimates per day). Each MAD datum (rounded to the nearest integer) for any given species, year and site was then matched to the pertaining DDi data (see electronic supplementary material for analyses showing high correlation among DDi computed at various temperature thresholds). Thus, species-specific DDi for any given year and site is the sum of temperatures above threshold i from 1 January until MAD.
(c). Coding of species migration strategy, population trends, habitats and diet
Species were classified either as SDM or LDM migrants according to Cramp [38] (see electronic supplementary material).
Population trends for 1990–2000 were obtained from BirdLife International [34] for the countries where most of the populations migrating through the study sites breed (Denmark, Norway, Sweden, Lithuania, Latvia, Estonia and Finland). We excluded Russia because trends for this country refer to an area extending eastwards to the Ural Mountains, which has limited relevance for birds migrating through the study sites. In the original source, trends for each country and species are reported either as accurate trends (% change) or as trend classes (e.g. 20–29% change). We expressed trends as 11 trend classes, from −5 to +5 (0: no change; 1: <20% change; 2: 20–29% change; 3: 30–49% change; 4: 50–79% change; 5: ≥80% change) [39]. Trends are classified according to three levels of data quality (i.e. trends based on qualitative, qualitative/quantitative or quantitative data; [34]). We considered only trend estimates based on qualitative/quantitative and quantitative data. Trend classes for each species were calculated as the mean trend class across the countries for which an estimate was available. For five species, trend estimates were not available. In addition, we used trend estimates from other sources for Denmark [40], Sweden [41] and Finland [35] (see electronic supplementary material).
The analyses that were run on mean population trends as derived from BirdLife International [34] were also run on population trends for Finland derived from the same source or from another source [35] in relation to the species-specific mean linear regression coefficient (DDiYr) of DDi at MAD on year recorded at Jurmo and Hanko, because these two sites are located in southern Finland, and MAD recorded there mainly refer to birds breeding in Finland [42]. Conversely, phenological data recorded at Helgoland and Rybachy cannot be associated to specific countries.
The main habitat of individual species was classified according to BirdLife International [34] as farmland versus non-farmland or as aquatic versus non-aquatic, because farmland and non-aquatic species have declined more than species from other habitats [43–47]. Because species at different trophic levels may differ in response to climate change, diet was also factored out in the analyses. Species were classified as mainly primary consumers, secondary/higher level consumers or omnivorous according to Cramp [38]. Finer-scale classification of diet in nine classes led to similar results (details not presented).
(d). Comparative analyses and phylogenetic information
In comparative studies, species cannot be considered as statistically independent because of their shared phylogenetic history. To control for the effects of common descent in the analyses of population trends, we calculated independent linear contrasts [48,49] using the CAIC library of the software R 2.8.1 [50]. Contrasts were analysed by regression analyses through the origin [49]. The sources of phylogenetic information are reported in the electronic supplementary material.
(e). Statistical analyses
Analyses reported in the main text, as well as those reported in the electronic supplementary material, were based on standard t-tests and on linear models or linear mixed models. In linear mixed models, species and site were included as random effect factors, whereas migration strategy was considered as a fixed factor. The analyses of mean population trends were run by weighted least-squares regression models using the number of countries for which population trend estimates were available as a weighting factor.
3. Results and discussion
(a). Temporal variation in arrival dates and degree-days at arrival
Owing to the rise in winter and spring temperatures, at all four study sites spring DD0 showed a marked increase since 1958 (figure 2). Around 30 April, when bulk migration concentrates, average DD0 between 1989 and 2009 was as much as 58 per cent larger than in the previous three decades. However, migrants have not kept track of this change. In fact, linear regression coefficients (DD0Yr) of DD0 recorded at MAD for individual species and springs on year were positive for 97.1 per cent of the 242 phenological time series, and 109 (=45.0%) of them were significantly larger than 0. When included in polynomial regression models to account for nonlinear trends in change of DD0, the squared term of year never attained significance (p > 0.05 in all cases). Mean DD0Yr (=2.66°C yr−1 (0.099 s.e.m.)) computed across all time series was significantly larger than 0 (one-sample t241 = 26.97, p < 0.001, n = 242 series; figure 3a). The mean species-specific DD0Yr values were also positive in 97.4 per cent of the cases and their mean (2.70°C yr−1 (0.136 s.e.m.)) was significantly larger than 0 (t116 = 19.82, p < 0.001, n = 117 species; figure 3b). These results imply that migrants arrive at larger DD0 now than in the past, therefore showing that they have accumulated a ‘thermal delay’ upon arrival.
Figure 3.
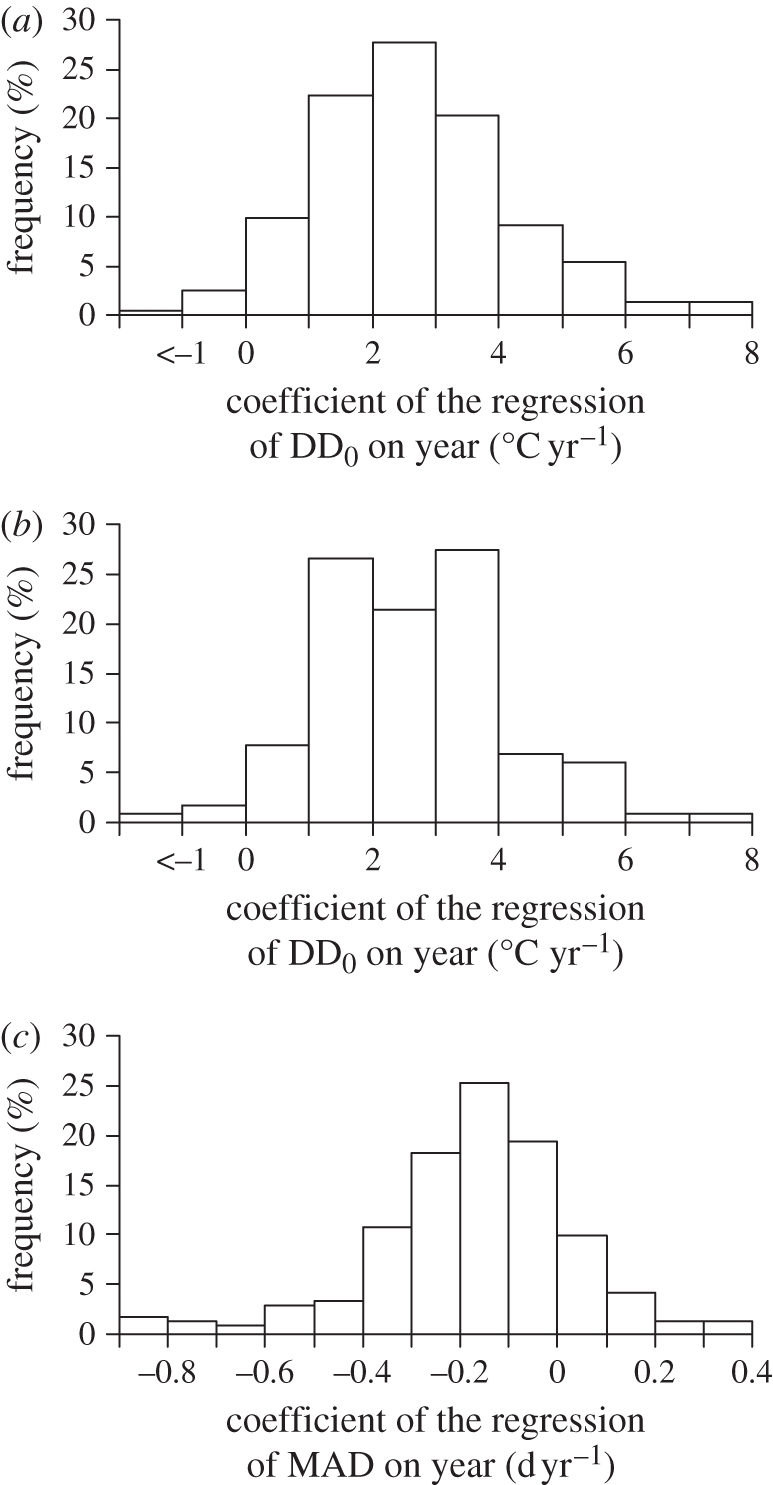
Temporal variation in DD0 at arrival, and in arrival date. Frequency distribution of DD0Yr for (a) phenological time series or (b) within-species means. (c) Frequency distribution of the mean species-specific MADYr.
During the same period, in 83.5 per cent of the 242 time series the linear regression coefficient (MADYr) of MAD on year was smaller than 0, and significantly so (p < 0.05) in 100 (=41.3%) of the cases, whereas in only seven cases it was significantly positive. Hence, most time series showed earlier arrival of migrants in recent years. On average, MADYr values were significantly smaller than 0 (mean = −0.166 d yr−1 (0.0138 s.e.m.); t241 = −12.02, p < 0.001, n = 242). The mean within-species values of these phenological trends were negative for 88 per cent of the species and their mean (−0.162 d yr−1 (0.0216 s.e.m.)) was also significantly negative (t116 = −7.49, p < 0.001, n = 117 species; figure 3c). Thus, advancement of arrival date may have not been sufficient to compensate for climate change (figure 1), as DD0 at arrival have increased, and birds are now arriving at later spring phenophases.
(b). Arrival dates and degree-days at arrival of SDMs or LDMs
LDM species have advanced their arrival less than SDM (mean MADYr, estimated by averaging the species means computed across sites, for LDM = −0.073 d yr−1 (0.0252 s.e.m.); SDM = −0.214 d year−1 (0.0294 s.e.m.); two-samples t115 = 3.25, p = 0.002, n = 43 LDM and 74 SDM). Both LDM and SDM species have suffered a thermal delay at MAD, as the mean value of the mean species-specific DD0Yr was significantly larger than 0 for both type of migrants (SDM: one-sample t73 = 13.92, p < 0.0001; LDM: t42 = 19.31, p < 0.0001). However, smaller advancement of MAD by LDM than SDM has translated into larger mean DD0Yr values for the former, implying that they have accumulated larger thermal delay than SDM (mean DD0Yr for LDM = 3.62°C yr−1 (0.188 s.e.m.); SDM = 2.17°C yr−1 (0.156 s.e.m.); two-samples t115 = 5.85, p < 0.001, n = 43 LDM and 74 SDM).
When run on DD−3 or DD+3, all the above analyses led to results similar to those obtained using DD0. Moreover, the analyses of DDiYr or MAD based on linear mixed models with species and site as random factors and migration strategy as a fixed effect also led to qualitatively identical results (see electronic supplementary material).
(c). Thermal delay and population trends
As inability of birds to track climate change may cause their populations to decline [3,51], we predicted that species with larger ‘thermal delay’ at arrival have experienced larger population declines. By using population trends reported for 1990–2000 in BirdLife International [34] for migrants breeding in seven countries north of the study sites, we found this prediction to be supported (table 1 and figure 4a). To check for robustness of this result, we first tested if DD0Yr at the Finnish sites of Hanko and Jurmo alone (which mostly refer to birds breeding in Finland) negatively predicted population trends in that country, and again found that this was the case (table 1 and figure 4b). Moreover, estimates of population trends in Finland during 1983–2005 [35] were also found to negatively covary with DD0Yr recorded on that time interval at Hanko and Jurmo (table 1 and figure 4c).
Table 1.
Population trends in relation to DD0Yr (regression coefficient of DD0 at MAD on year). The effects of DD0Yr on population trends are obtained from a model including a binary factor migration strategy but excluding the effect of the non-significant interaction. Full models are reported in electronic supplementary material, tables S1 and S2. Finland DD0Yr data were averaged between Hanko and Jurmo. Population trends for the seven countries and for Finland (a) are derived from BirdLife International [34]. Population trends used in model Finland (b) are derived from Väisänen [35]. The number of short-distance or long-distance migrants for which population trend data were available was 70 and 42 for the seven countries, 65 and 39 for Finland (a), and 35 and 27 for Finland (b).
| t | d.f. | p | estimate | |
|---|---|---|---|---|
| species-specific data | ||||
| seven countries | −2.30 | 109 | 0.023 | −0.222(0.097) |
| Finland (a) | −2.39 | 101 | 0.019 | −0.379(0.159) |
| Finland (b) | −2.53 | 59 | 0.014 | −0.654(0.258) |
| independent contrasts | ||||
| seven countries | −2.72 | 106 | 0.008 | −0.221(0.081) |
| Finland (a) | −3.13 | 98 | 0.002 | −0.458(0.146) |
| Finland (b) | −2.90 | 58 | 0.005 | −0.753(0.259) |
Figure 4.
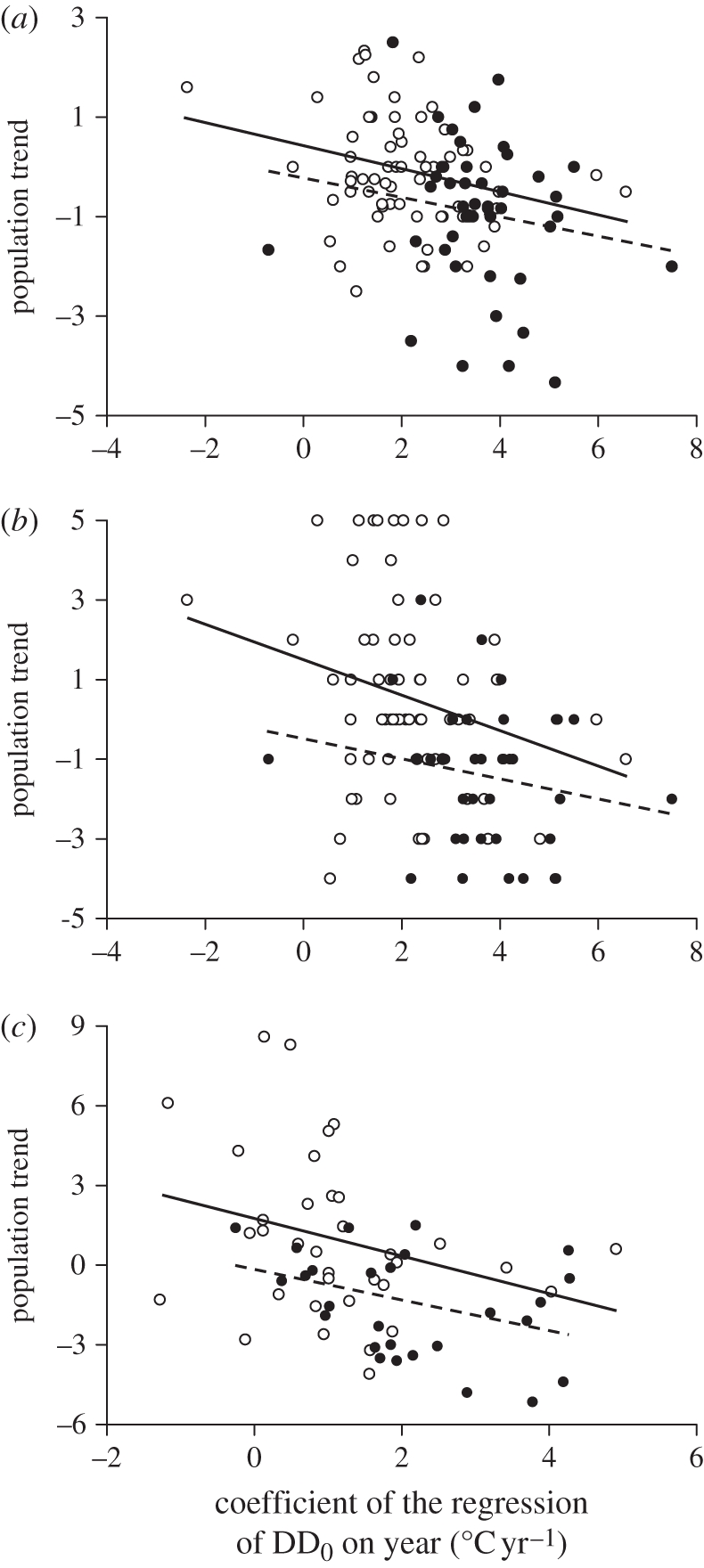
Population trends in relation to thermal delay. (a) Mean species population trends [34] in the seven countries in relation to species DD0Yr. (b) Population trends in Finland [34] in relation to species DD0Yr computed for the Finnish sites (Jurmo and Hanko). (c) Population trends in Finland obtained from a different source [35] in 1983–2005 in relation to species DD0Yr calculated over the same period for the Finnish sites. Regression lines fitted to short-distance (empty circles, solid line) or long-distance (filled circles, dashed line) migrants show that the slopes are similar.
These results and the fact that population trends during 1990–2000 are positively correlated with those recorded over longer time periods at continental as well as national scales [39] (see electronic supplementary material) show that increased DD0 at arrival negatively predicts population trends. Analyses where we controlled for ecological variation among species in terms of habitat and trophic level, and/or for similarity in response owing to common ancestry, led to consistent results (table 1; see electronic supplementary material). Moreover, analyses run on DD−3Yr or DD+3Yr both on ‘raw’ species data and while controlling for phylogenetic effects largely confirmed the results of analyses based on DD0Yr (see electronic supplementary material, tables S1 and S2).
Several previous studies of migratory birds have provided evidence that migrants have advanced their arrival to the breeding areas during recent decades [19,20]. Conversely, very few studies have investigated whether the rate of change in migration and breeding phenology has compensated for generalized phenological shifts owing to climate change [10,11,16]. Here, we have adopted an approach based on degree-days accumulation to investigate whether the progress of spring at the time when migratory birds arrive to their breeding areas has changed over the last five decades. This approach is novel and can be extended to studies of the ecological consequences of phenological response to climate change in studies of birds and other taxa, owing to the ubiquitous nature of the effects of temperature on the phenology of plants and ectothermic organisms (see §1).
Advancement in arrival date to the breeding areas, which has indeed occurred for most species, has not fully compensated for climate change, and larger degree-day values at arrival in recent years may imply that birds have become ecologically mismatched. This is the case because the phenophases of plants and ectothermic animals, including those relevant to bird ecology, are mostly regulated by temperature [24–29,52]. The present study thus rests on the assumption that long-term increase in degree-days at arrival causes an (increasing) ecological mismatch. This would not be the case if birds were arriving too early (i.e. at too low degree-days) in the past, as an increase in degree-days at arrival would ensure smaller, rather than larger, ecological mismatch. Although no unequivocal reference for optimal conditions (in terms of degree-days at arrival) is known for any bird species, we consider the possibility that ecological matching increases with increasing degree-days at arrival a remote one. First, selection for earlier arrival and breeding has been repeatedly documented in bird studies [17], implying that birds arrive on average too late, rather than too early. Second, birds are advancing their arrival date, whereas they should be expected to delay it if they were arriving too early. Third, temperature changes have been occurring at faster pace during recent decades than in the previous ones [4] (see also figure 2). If birds show a constant latency in responding to climate change, more rapid recent climate change per se justifies the expectation of larger ecological mismatch. We thus consider the assumption that higher degree-days at arrival are associated with stronger ecological mismatch as warranted. Obviously, the extent of ecological mismatch arising because of a unit increase in degree-days may widely vary among species according to their trophic level and habitat. However, we found no statistical evidence that the effect of DDiYr on population trend depended on diet or habitat, suggesting that any negative consequence of change in degree-days at arrival on population trends was independent of variation in major ecological traits of the species.
The ability of birds to cope with climate change by adjusting their migration phenology may be constrained by the timing of life-cycle phases preceding migration, which may limit the scope for phenotypically plastic response, by different climatic variation in the wintering areas and en route, and/or by depleted genetic variance in migration traits, which can hinder microevolutionary response [14]. All these scenarios are compatible with the observation that LDMs, having advanced their arrival less, have accumulated a larger ‘thermal delay’ when compared with SDMs.
We have also shown that species with larger ‘thermal delay’ at arrival have undergone the largest population decline, while species with small-to-moderate thermal delay have barely shown a decline. Previous studies have shown that LDMs have declined more than SDMs [39]. Although this difference may also be caused by factors operating differently on their wintering grounds or along the migration route, the present results suggest that the larger thermal delay they have accumulated may contribute to their larger decline.
Hence, ecological mismatch at arrival from spring migration may be a general condition among birds breeding in northern Europe, and failure to adjust phenological events to rapidly changing climatic conditions may be an important factor causing negative demographic and conservation effects.
Acknowledgements
We thank A. P. Møller, C. Both and two anonymous reviewers for constructive comments on an earlier version of the paper.
References
- 1.Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J.-N., Høgh-Guldberg O., Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 3.Møller A. P., Rubolini D., Lehikoinen E. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16 195–16 200 10.1073/pnas.0803825105 (doi:10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IPCC 2007. Climate change 2007: the physical science basis. Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 6.Böhning-Gaese K., Lemoine N. 2004. Importance of climate change for the ranges, communities and conservation of birds. Adv. Ecol. Res. 35, 211–236 10.1016/S0065-2504(04)35010-5 (doi:10.1016/S0065-2504(04)35010-5) [DOI] [Google Scholar]
- 7.Menzel A., et al. 2006. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 10.1111/j.1365-2486.2006.01193.x (doi:10.1111/j.1365-2486.2006.01193.x) [DOI] [Google Scholar]
- 8.Schwartz M. D., Ahas R., Aasa A. 2006. Onset of spring starting earlier across the Northern Hemisphere. Glob. Change Biol. 12, 343–351 10.1111/j.1365-2486.2005.01097.x (doi:10.1111/j.1365-2486.2005.01097.x) [DOI] [Google Scholar]
- 9.Klein Tank A. M. G., et al. 2002. Daily dataset of 20th-century surface air temperature and precipitation series for the European climate assessment. Int. J. Climatol. 22, 1441–1453 10.1002/joc.773 (doi:10.1002/joc.773) [DOI] [Google Scholar]
- 10.Visser M. E., Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569 10.1098/rspb.2005.3356 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Both C., van Asch M., Bijlsma R. G., van den Burg A. B., Visser M. E. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations. J. Anim. Ecol. 78, 73–83 10.1111/j.1365-2656.2008.01458.x (doi:10.1111/j.1365-2656.2008.01458.x) [DOI] [PubMed] [Google Scholar]
- 12.Gienapp P., Leimu R., Merilä J. 2007. Responses to climate change in avian migration time: microevolution versus phenotypic plasticity. Clim. Res. 35, 25–35 10.3354/cr00712 (doi:10.3354/cr00712) [DOI] [Google Scholar]
- 13.Pulido F. 2007. Phenotypic changes in spring arrival: evolution, phenotypic plasticity, effects of weather and condition. Clim. Res. 35, 5–23 10.3354/cr00711 (doi:10.3354/cr00711) [DOI] [Google Scholar]
- 14.Møller A. P., Fiedler W., Berthold P. (eds) 2010. Effects of climate change on birds. Oxford, UK: Oxford University Press [Google Scholar]
- 15.Møller A. P., Fiedler W., Berthold P. (eds) 2004. Advances in ecological research: birds and climate change. Oxford, UK: Elsevier Academic Press [Google Scholar]
- 16.Charmantier A., McCleery R. H., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 10.1126/science.1157174 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 17.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press [Google Scholar]
- 18.Visser M. E., van Noordwijk A. J., Tinbergen J. M., Lessells C. M. 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 10.1098/rspb.1998.0514 (doi:10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 19.Lehikoinen E., Sparks T. H., Zalakevicius M. 2004. Arrival and departure dates. Adv. Ecol. Res. 35, 1–31 10.1016/S0065-2504(04)35001-4 (doi:10.1016/S0065-2504(04)35001-4) [DOI] [Google Scholar]
- 20.Rubolini D., Møller A. P., Rainio K., Lehikoinen E. 2007. Intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Clim. Res. 35, 135–146 10.3354/cr00720 (doi:10.3354/cr00720) [DOI] [Google Scholar]
- 21.Saino N., Szép T., Romano M., Rubolini D., Spina F., Møller A. P. 2004. Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol. Lett. 7, 21–25 10.1046/j.1461-0248.2003.00553.x (doi:10.1046/j.1461-0248.2003.00553.x) [DOI] [Google Scholar]
- 22.Jonzén N., et al. 2006. Rapid advance of spring arrival dates in long-distance migratory birds. Science 312, 1959–1961 10.1126/science.1126119 (doi:10.1126/science.1126119) [DOI] [PubMed] [Google Scholar]
- 23.Both C., Visser M. E. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 10.1038/35077063 (doi:10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- 24.Podolski A. S. 1984. New phenology. New York, NY: Wiley [Google Scholar]
- 25.Wielgolaski F. E. 1999. Starting dates and basic temperatures in phenological observations of plants. Int. J. Biometeorol. 42, 158–168 10.1007/s004840050100 (doi:10.1007/s004840050100) [DOI] [Google Scholar]
- 26.Menzel A. 2003. Plant phenological anomalies in Germany and their relation to air temperature and NAO. Clim. Change 57, 243–263 10.1023/A:1022880418362 (doi:10.1023/A:1022880418362) [DOI] [Google Scholar]
- 27.Schwartz M. D. (ed.) 2003. Phenology. Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 28.Trudgill D. L., Honek A., Li D., Van Straalen N. M. 2005. Thermal time: concepts and utility. Ann. Appl. Biol. 146, 1–14 10.1111/j.1744-7348.2005.04088.x (doi:10.1111/j.1744-7348.2005.04088.x) [DOI] [Google Scholar]
- 29.Nietschke B. S., Magarey R. D., Borchert D. M., Calvin D. D., Jones E. 2007. A developmental database to support insect phenology models. Crop Prot. 26, 1444–1448 10.1016/j.cropro.2006.12.006 (doi:10.1016/j.cropro.2006.12.006) [DOI] [Google Scholar]
- 30.Bradshaw W. E., Holzapfel C. M. 2006. Evolutionary response to rapid climate change. Science 312, 1477–1478 10.1126/science.1127000 (doi:10.1126/science.1127000) [DOI] [PubMed] [Google Scholar]
- 31.Harrington R., Woiwod I., Sparks T. 1999. Climate change and trophic interactions. Trends Ecol. Evol. 14, 146–150 10.1016/S0169-5347(99)01604-3 (doi:10.1016/S0169-5347(99)01604-3) [DOI] [PubMed] [Google Scholar]
- 32.Shamoun-Baranes J., van Loon E., Alon D., Alpert P., Yom-Tov Y., Leshem Y. 2006. Is there a connection between weather at departure sites, onset of migration and timing of soaring-bird autumn migration in Israel? Global Ecol. Biogeogr. 15, 541–552 10.1111/j.1466-8238.2006.00261.x (doi:10.1111/j.1466-8238.2006.00261.x) [DOI] [Google Scholar]
- 33.Hüppop O., Hüppop K. 2003. North Atlantic Oscillation and timing of spring migration in birds. Proc. R. Soc. Lond. B 270, 233–240 10.1098/rspb.2002.2236 (doi:10.1098/rspb.2002.2236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.BirdLife International 2004. Birds in Europe: population estimates, trends and conservation status. Cambridge, UK: BirdLife International [Google Scholar]
- 35.Väisänen R. A. 2006. Maalinnuston kannanvaihtelut Etelä- ja Pohjois-Suomessa 1983–2005. Linnut-vuosikirja 2005, 83–98 [Google Scholar]
- 36.Sokolov L. V., Markovets M. Y., Shapoval A. P., Morozov Y. G. 1998. Long-term trends in the timing of spring migration of passerines on the Courish Spit of the Baltic Sea. Avian Ecol. Behav. 1, 1–21 [Google Scholar]
- 37.Vähätalo A. V., Rainio K., Lehikoinen A., Lehikoinen E. 2004. Spring arrival of birds depends on the North Atlantic Oscillation. J. Avian Biol. 35, 210–216 [Google Scholar]
- 38.Cramp S. 1998. The complete birds of the western Palearctic on CD-ROM. Oxford, UK: Oxford University Press [Google Scholar]
- 39.Sanderson F. J., Donald P. F., Pain D. J., Burfield I. J., van Bommel F. P. J. 2006. Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 131, 93–105 10.1016/j.biocon.2006.02.008 (doi:10.1016/j.biocon.2006.02.008) [DOI] [Google Scholar]
- 40.Heldbjerg H., Eskildsen A. 2009. Overvågning af de almindelige fuglearter i Danmark 1975–2008. Årsrapport for Punkttællingsprojektet. Svendborg, Denmark: Dansk Ornitologisk Forening [Google Scholar]
- 41.Lindström Å., Green M., Ottvall R., Svensson S. 2009. Övervakning av fåglarnas populationsutveckling. Årsrapport för 2008. Lund, Sweden: Lunds Universitet, Ekologiska Institutionen [Google Scholar]
- 42.Tottrup A., Thorup K., Rainio K., Yosef R., Lehikoinen E., Rahbeck C. 2008. Avian migrants adjust migration in response to environmental conditions en route. Biol. Lett. 4, 685–688 10.1098/rsbl.2008.0290 (doi:10.1098/rsbl.2008.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker G., Heath M. 1994. Birds in Europe: their conservation status. Cambridge, UK: BirdLife International [Google Scholar]
- 44.Fuller R. J., Gregory R. D., Gibbons D. W., Marchant J. H., Wilson J. D., Baillie S. R., Carter N. 1995. Population declines and range contractions among lowland farmland birds in Britain. Conserv. Biol. 9, 1425–1441 10.1046/j.1523-1739.1995.09061425.x (doi:10.1046/j.1523-1739.1995.09061425.x) [DOI] [Google Scholar]
- 45.Tiainen J., Pakkala T. 2000. Population changes and monitoring of farmland birds in Finland. Linnut-vuosikirja 1999, 98–105 [Google Scholar]
- 46.Wretenberg J., Lindström Å., Svensson S., Pärt T. 2007. Changes in local species richness of farmland birds in relation to land-use and landscape structure. J. Appl. Ecol. 44, 933–941 10.1111/j.1365-2664.2007.01349.x (doi:10.1111/j.1365-2664.2007.01349.x) [DOI] [Google Scholar]
- 47.Both C., van Turnhout C. A. M., Bijlsma R. G., Siepel H., van Strien A. J., Foppen R. P. B. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 1685, 1259–1266 10.1098/rspb.2009.1525 (doi:10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 10.1086/284325 (doi:10.1086/284325) [DOI] [Google Scholar]
- 49.Purvis A., Rambaut A. 1995. Comparative analysis by independent contrasts (CAIC). Comp. Appl. Biosci. 11, 247–251 [DOI] [PubMed] [Google Scholar]
- 50.R Development Core Team 2008. R: A language and environment for statistical computing, version 2.8.1. Vienna, Austria: R Foundation for Statistical Computing; http://www.r-project.org [Google Scholar]
- 51.Both C., Bouwthuis S., Lessells C. M., Visser M. E. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 10.1038/nature04539 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 52.Eeva T., Lehikoinen E., Lummaa V., Rönkä M., Currie D. 2002. Different responses to cold weather in two pied flycatcher populations. Ecography 25, 705–713 10.1034/j.1600-0587.2002.250606.x (doi:10.1034/j.1600-0587.2002.250606.x) [DOI] [Google Scholar]