Abstract
Predation selects for numerous traits in many animal species, with sick or parasitized prey often being at high risk. When challenged by parasites and pathogens, prey with poor immune functions are thus likely to be at a selective disadvantage. We tested the hypothesis that predation by birds selects for increased immune function in a wild population of male damselflies Calopteryx splendens, while controlling for a trait known to be under selection by bird predation, dark wing-spots. We found that selection on both immune function and wing-spot size was significantly positive, and that selection on either trait was independent of selection on the other. We found no evidence of nonlinear quadratic or correlational selection. In contrast to previous studies, we found no phenotypic correlation between immune function and wing-spot size. There was also no difference in immune response between territorial and non-territorial males. Our study suggests that predation may be an important agent of selection on the immune systems of prey, and because the selection we detected was directional, has the potential to cause phenotypic change in populations.
Keywords: condition-dependent, encapsulation, immunity, individual quality, selection gradient, sexual ornaments
1. Introduction
Predation is a key agent of natural selection [1] and shapes the evolution of many characters in prey populations [2]. Predators are often more likely to kill prey that are in poor condition (e.g. [3,4]), such as those that are heavily parasitized [4]. Variation between individuals in their ability to mount an immune response to pathogens or parasites is thus likely to affect their likelihood of being killed by predators. However, the nature of such a ubiquitous agent of selection as predation on the immune system of any prey species has received no direct attention to date.
The nature and strength of selection by predation on any component of the immune system in any prey species is unknown. There is a general premise in life-history theory that maintaining the immune system [5] and avoiding predators [6,7] is costly, and also condition-dependent (e.g. [8,9]). Moreover, predators may select traits in prey correlated with their immune function [10]. Natural selection on immune defence via predation therefore has the potential to be directional, through condition dependence, stabilizing, when the costs of increased immune function are high, or correlational, if predators are selecting prey traits correlated with variance in their immune function. We set out to answer two questions regarding predation as an agent of selection on the immune function of wild male damselflies, Calopteryx splendens. (i) Is predation a significant agent of selection on the immune system of wild C. splendens? (ii) Is selection linear (directional) or nonlinear (stabilizing or correlational)?
We chose to study C. splendens because predation by birds is a known agent of selection on male wing morphology in some populations [11]. Males have melanized wing patches and those with lighter ‘wing-spots’, and wings of intermediate length and width, are least likely to be killed. This is possibly owing to darker wing-spots being more conspicuous to predators, and longer and wider wings being favoured for aerial speed and manoeuvrability, respectively [11]; intermediate wing length and width are thus favoured because of a positive correlation between all three traits. Males with dark wing-spots survive only if they have long wings to compensate for their increased conspicuousness [11]. Moreover, stabilizing selection for wing length results in indirect correlational selection favouring wing-spots of intermediate length [11]. However, males with large wing-spots achieve the highest mating success [12] and have the highest levels of immunocompetence [13,14]. In damselflies, the expression of male wing-spots [15] and immune function [16,17] are often stated as being condition-dependent. Because wing-spots and components of immunity such as the ability to encapsulate parasites both depend on the production of melanin that is likely to be costly, wing-spot size and this aspect of immune function may be negatively correlated. There is thus potential for predation to result in linear and nonlinear selection on the encapsulation component of the immune system of male C. splendens. We therefore studied a Finnish C. splendens population and measured selection via bird predation on this specific component of the immune function of males, while also taking selection on their wing-spots into account.
2. Material and methods
(a). Study species, study site and insect collection
Calopterygids are large, broad-winged damselflies that have metallic-coloured bodies and wing veins. Calopteryx splendens is widely distributed throughout Europe and western Asia. In western Europe, C. splendens is often abundant near to the slow-flowing streams and small rivers it favours. The males are territorial and defend patches of floating vegetation, which females use as oviposition sites [18]. The sexes are dimorphic. Males have green heads and thoraxes, blue abdomens, and a distinctive dark band on each wing. Females are all green and have clear wings.
From 5 June to 11 July 2007, we studied the C. splendens population of the Tarvasjoki River (60°32′ N, 22°45′ W) near Turku, in southwest Finland. We used a 440 m section of this small river. The study section consisted of a 90 m long rocky rapid and 350 m of smoothly flowing water, and was relatively isolated because the river immediately upstream and downstream had no suitable habitat for C. splendens. We further sub-divided the study section by placing wooden sticks 5 m apart to enable us to record the location of each C. splendens male. On two separate days (5 June and 13 June), we collected all male C. splendens which we were able to find along the banks of the study section of river and in the adjoining meadows with a butterfly net. Immediately after capture, each insect was placed into a 48 ml (75 mm in height and 45 mm in diameter) transparent cylindrical plastic vial. All vials were then placed into a cool box before being taken to the laboratory.
(b). Laboratory procedures
In the laboratory, we estimated the age of each insect using a five-point age class scale modified slightly from that described by Siva-Jothy & Plaistow [19]. Age class in odonates can be determined by the stiffness of, and wear to, the leading edge of their wings, both of which increase with age. The age class scale we used ranged from class zero, which were freshly emerging individuals, to class four, which were damselflies nearing the end of their lives characterized by hard and broken wings. Before release, each insect was marked on both hind wings using a unique three-letter code with a silver marker pen. We acknowledge that marking the wings may have had the potential to affect predation risk, and also damselfly behaviour. For instance, this may have made the insects more prone to attack by predators, and/or affected their mating success or territorial behaviour, if the ink made it easier for them to be seen by predators and/or conspecifics. However, this method was suitable for our study for three reasons. (i) All individuals were marked similarly, thus controlling for any variation in any effects of marking between individuals. (ii) Alternative marking methods, e.g. coloured dots on the abdomen or a numbered marker glued to the thorax, have the same potential to affect predation and/or behaviour as does ink marking. Moreover, these methods were unsuitable because this study ultimately relied on the retrieval and identification of the wings of each insect (see below). (iii) Variation in immune system response is likely to affect the likelihood of predation and behavioural variation in male C. splendens over and above any potential effects of the same ink marking methods used in this study (see [20,21]). Moreover, we saw marked males defending their territories and mating with females normally, suggesting that they were behaving typically and that females accepted them as mates.
To control for male age, we only used class two individuals for treatment, which had wings that were undamaged and were still dorso-ventrally flexible along their complete length [19]. At the time of marking, we also measured to the nearest 0.01 mm the total length of each hind wing, and the length of the spot on the same wings, with digital Vernier callipers (see [22]), and weighed each insect to the nearest 0.01 g. Because the main purpose of this study was to examine the effects of predation on the immune function of the insects, and to reduce the time we spent handling each animal, we did not take multiple measurements associated with variation in wing-spots, e.g. width, shape and pigmentation. This was to minimize the potential effects handling may have had on the insects that could have affected their subsequent predation risk or behaviour. Although body mass may change over time in odonates, each insect was also weighed once during the study, immediately after the marking of its wings. This was because the repeated capture and weighing of each insect throughout its lifetime was impractical, and would increase the potential for handling to affect the main results.
A standard technique for initiating and measuring immune responses in odonates, and in other insects, is the insertion of a small artificial object, e.g. a nylon monofilament, through the cuticle of an individual insect, and then quantifying the degree of melanization over a set time (e.g. [13,17,23]). Damselflies defend themselves against infection by ectoparasitic water mites by encapsulating the feeding tubes of the mites [24,25]. The ability of insects to encapsulate abiotic material has shown to be related to their ability to encapsulate parasites [26,27] and to resist fungal pathogens [28], which suggests that the use of this method to measure an individual's immune function is biologically valid.
For each of the 157 age class two individuals caught, we measured its ability to mount an immune response by first inserting a single, sterile 2 mm length of nylon monofilament (diameter 0.18 mm, rubbed with fine sandpaper then knotted) into its fourth abdominal pleura on the dorsal side of the sternal–tergal margin (see also [13,23]). Each insect was then placed into a cylindrical plastic vial that prevented excessive movement, and left for 20 h at constant room temperature (22°C). This time period was used so each insect's immune system could react to the implant, which takes at least 5 h ([13]; see also [29]), and due to the logistical necessity of subsequently releasing all of the animals during daylight hours while minimizing any handling. Each implant was then gently removed from the insect and frozen for later analysis. All insects were then taken back to the river and released at their point of capture. We also acknowledge that this handling of the insects may have affected their subsequent behaviour, although the normal behaviour demonstrated by the insects after release (see above) suggests this was unlikely (M. J. Rantala, J. Honkavaara & J. Suhonen 2007, personal observations). This level of handling was unavoidable because of the time needed for the process to be successful, and because recapture and further manipulation for implant removal in the field was likely to have a more negative effect on each insect than our chosen method. It should be noted that as with our marking method, any potential effects of handling for immune system measurements were controlled for in the study because all insects were treated identically (see above).
After removal from the insect, each implant was photographed from two different angles under a light microscope with a digital camera. The pictures were then analysed using the computer program Image Pro. As a measure of encapsulation rate, we used the mean of the grey values of reflecting light from the two digital pictures of each implant. The data were transformed by subtracting the observed grey values from a control value. The control value was obtained by photographing a haphazardly selected new, clear implant. The darkest grey values thus corresponded to the highest encapsulation rates. The repeatability of this method is high [30].
(c). Measuring predation, reproductive lifespan and territoriality
The white wagtail Motacilla alba alba is a major predator of Calopteryx damselflies in riparian areas [11,31]. An M. alba alba individual will return to the same few points to kill and process its prey after capture and before eating. At these ‘slaughter stations’ [11], a bird will remove and discard the wings of the damselflies it catches. The study stretch of river contained at least six M. alba alba, which had 13 recognizable slaughter stations. After the insects were released, each slaughter station was checked daily for the C. splendens wings the birds had discarded. This enabled us to record which of the marked insects the birds had killed, and when they had killed them, by examination of the marks on each discarded wing. All damselfly wings were removed daily from each slaughter station.
We also patrolled the riverbanks and the nearby meadows three times daily: once in the morning, once at noon, and again in the afternoon. This was done between 10.00 and 16.00 h to coincide with the maximum activity of the damselflies [32]. On cool or damp days, we only checked for damselflies once a day. We counted all marked males still present and recorded the location of each one on the riverbank to the nearest metre. The activation of the immune system by a nylon monofilament has found to induce male C. virgo to disperse up to 1.5 km in same study river [21]. At least every other day, we therefore checked all suitable habitats within a 3-km radius from our study area for any marked individuals but found none. This suggests that dispersal in male C. splendens is less likely to occur than in C. virgo males (see also [20]) and that increased dispersal by some individuals away from the study area was unlikely to have affected our results. The wings of the last individual known to have been killed by the wagtails were found on the 24 June. The last day any marked individual was seen was on 29 June, with the study finishing on the 6 July, after several days of subsequent searching after the last marked male was seen alive. Our data are thus based on ‘mark–release–recapture’ data under the assumption that a male whose wings had not been found at any slaughter station and had not been seen for seven days was dead, and the cause of its death was not predation by any of the resident wagtails in the study area. This assumption is justified because in our data the ‘recapture’ probability (repeated sightings of marked individuals) was high (see also [20,33]). Because all marked C. splendens were of equal age and we checked their survival status daily, we could subsequently calculate their reproductive lifespans as the number of days between release and the last time they were seen alive or known to have been killed by the wagtails (see [34]). During the observation periods, we also monitored the behaviour of each marked male for several minutes several times during the day using binoculars to determine if it was territorial or not. A male was categorized as being territorial if it was seen to defend a territory and not to change its location during the day, because a male damselfly may not necessarily be territorial throughout its entire reproductive lifespan [35–37]. If males are territorial they will stay close to a given area by the riverside near to floating vegetation, and will demonstrate aggression to intra- and interspecific males [33,38]. They will also often fly close to the surface of the water in their territory for several seconds before alighting near to where they took off.
(d). Statistical analysis
To identify the nature and strength of selection, we calculated linear, quadratic and correlational selection gradients using the regression methods of Lande & Arnold [39]. This uses least-squares regression coefficients as fitness functions for selection acting on each trait. After first standardizing all measurements to the standard deviation of each trait to account for differences in measurement units, we initially performed a single multiple least-squares regression consisting of the four linear terms predicting survival (1, survived the predation threat by the resident wagtails; 0, killed by the resident wagtails). Because our measure of fitness was categorical, we used binary logistic regression to test the significance of each partial regression coefficient [40]. We then added the quadratic terms for the four traits, and the six cross-product terms, to fit the full nonlinear response surface to the model. For this part of the analysis, we used the GLM function in PASW Statistics 18 software for the Macintosh computer. The resulting quadratic coefficients and standard errors were doubled, because these measures equate to one-half the quadratic selection gradients outlined by Arnold and co-workers [39,41]. The inclusion of quadratic (to identify stabilising selection) and cross-product terms (to identify correlational selection) to a model will reduce its overall statistical power, and thus increases the chances of type II errors [42–44]. We therefore performed an additional canonical rotation of the response surface to identify the major axes of the nonlinear response surface using the RS REG function in SAS software [43]. This enabled us to identify the linear combinations of traits (eigenvectors, mn) along which selection was strongest.
Although we conducted a series of statistical tests to calculate selection gradients, there are two reasons why we do not present Bonferroni-corrected significance values as suggested by Kingsolver et al. [45]. (i) This would reduce statistical power to an unacceptable level, even by using a sequential method, thus increasing the likelihood of type II errors. (ii) Selection gradients are standardized estimates of effect size, which have been highlighted as being preferable to the presentation of a series of corrected significance values [46].
Wing length and body mass were both included as separate variables in our analyses for two reasons. (i) Wing length has been shown to be a target of selection by wagtails in another C. splendens population [11]. (ii) Immune function and wing-spots have both been shown to be condition-dependent in C. splendens males [13].
3. Results
(a). Selection via predation
The wings of 30 different males out of 157 (19%) were recovered from the 13 slaughter stations. The mean predation rate (±s.d.) was 0.051 (±0.061) individuals per day when there were more than 10 individuals alive. Two linear selection gradients were significantly positive: (i) encapsulation response and (ii) hind wing-spot size. Males with the highest encapsulation rates, and males with large wing-spots, were those most likely to survive the predation threat by the wagtails. Body mass and size (wing length) were not under significant selection (table 1). None of our nonlinear selection gradients was significantly different from zero (table 1). Moreover, our canonical analysis showed that it was unlikely that we made any type II errors by failing to detect any significant nonlinear selection with our regression analysis (table 2). This is because after we reduced our series of correlational selection gradients to a single canonical axis m1, λ is still small at only 0.024 and is not significantly different from zero. This is similar to our average correlational gradient of 0.02. Our average quadratic gradient is only −0.0075. Our data suggest that the selection we measured acting on both encapsulation rate and wing-spot size is positively directional, is not stabilizing or disruptive and is independent for both traits.
Table 1.
The four standardized linear selection gradients (β), and the matrix of the four standardized quadratic (γii), e.g. encapsulation × encapsulation; and the six correlational (γij), e.g. encapsulation × wing length, selection gradients.
|
γ |
|||||
|---|---|---|---|---|---|
| β ± s.e. | encapsulation | wing length | wing-spot | mass | |
| encapsulation | 0.07 ± 0.03* | −0.04 ± 0.06 | |||
| wing length | −0.03 ± 0.04 | 0.05 ± 0.04 | 0.06 ± 0.06 | ||
| wing-spot | 0.09 ± 0.03** | 0.02 ± 0.03 | 0.02 ± 0.04 | −0.04 ± 0.06 | |
| mass | −0.02 ± 0.04 | 0.01 ± 0.03 | 0.01 ± 0.04 | 0.02 ± 0.04 | −0.01 ± 0.06 |
*p = 0.027.
**p = 0.011.
Table 2.
The M matrix of eigenvectors of the response surface after the canonical rotation of γ.
|
M |
|||||
|---|---|---|---|---|---|
| eigenvalue (λ) | encapsulation | wing length | wing-spot | mass | |
| m1 | 0.024 | 0.492 | 0.758 | 0.310 | 0.295 |
| m2 | −0.007 | −0.106 | −0.375 | 0.238 | 0.890 |
| m3 | −0.024 | 0.190 | −0.337 | 0.854 | −0.347 |
| m4 | −0.040 | 0.843 | −0.413 | −0.344 | 0.018 |
(b). Correlations between variables
Contrary to our expectations, encapsulation response was not significantly correlated with wing length, wing-spot size, body mass (table 3) or with reproductive lifespan (rs = 0.01, p = 0.89, n = 157). As expected, heavier individuals had the longest wings and the largest wing-spots, and those males with long wings also had large wing-spots (table 3). However, when the standardized values of wing length (as an indicator of body size; b ± s.e. = 0.21 ± 0.09, t153 = 2.37, p = 0.019) and body mass (b ± s.e. = 0.12 ± 0.09, t153 = 1.39, p = 0.17) were used in a multiple least-squares regression predicting variation in wing-spot size, only wing length remained significant.
Table 3.
Descriptive statistics of, and correlation coefficients and covariances between, the four traits measured in the study. All correlations were Pearson's (r) except those involving encapsulation, which were Spearman's rank correlations (rs). Descriptive statistics and covariances are presented to aid future meta-analyses.
| mean, s.d, s.e | encapsulation | wing length | wing-spot | mass (mg) | ||
|---|---|---|---|---|---|---|
| encapsulation (%) | 52.80, 17.00, 1.35 | correlation | — | −0.064 | 0.033 | 0.095 |
| covariance | 289.0 | −0.878 | 0.942 | 18.8 | ||
| wing length (mm) | 29.32, 0.81, 0.15 | correlation | — | — | 0.259** | 0.448** |
| covariance | 0.655 | 0.354 | 4.25 | |||
| wing-spots (mm) | 14.56, 1.68, 0.13 | correlation | — | — | — | 0.212*** |
| covariance | 4.19 | |||||
| mass (mg) | 124.0, 11.7, 0.93 | correlation | — | — | — | — |
| covariance | 137.1 |
*p = 0.01.
**p < 0.001.
***p = 0.008.
None of the three size measurements was significantly correlated with reproductive lifespan (range of Spearman's rank correlation coefficients rs = −0.07 to 0.06, p = 0.22–0.49). There was no effect of male territorial behaviour on the likelihood of predation (χ2 test: 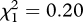 , p = 0.66), nor were there any significant differences between the means of any size trait or encapsulation rate between territorial or non-territorial males (range of t-values = 0.81–1.33, all p-values > 0.19). Territorial males (mean ± s.e. = 10.17 ± 0.77 days, n = 42) had longer reproductive lifespans than non-territorial males (5.23 ± 0.37 days, n = 115; Mann–Whitney u-test: Z = 5.59, p < 0.001).
, p = 0.66), nor were there any significant differences between the means of any size trait or encapsulation rate between territorial or non-territorial males (range of t-values = 0.81–1.33, all p-values > 0.19). Territorial males (mean ± s.e. = 10.17 ± 0.77 days, n = 42) had longer reproductive lifespans than non-territorial males (5.23 ± 0.37 days, n = 115; Mann–Whitney u-test: Z = 5.59, p < 0.001).
4. Discussion
Our study had three main findings. (i) Predation by wagtails generated positive directional selection on the encapsulation component of the immune function, and wing-spot size, of wild male C. splendens. (ii) The selection we measured at the time of the study was independent for each of the two traits. (iii) We found no evidence for nonlinear selection, either quadratic or correlational, on our chosen measure of immunity or for any other trait measured in the study. This is important because directional selection has historically been invoked as the strongest mechanism to produce phenotypic change in populations compared with nonlinear modes of selection [47], and suggests that predation can cause immune system change in C. splendens populations.
To our knowledge this is the first study to measure selection via predation on the immune system of any animal. Other studies have quantified selection on immune function in the wild but the selective agents have remained unidentified. For instance, Råberg & Stjernman [48] found that selection on the immune responses of blue tits Cyanistes caeruleus to tetanus and diptheria were directional and stabilizing, respectively. For male damselflies, Calopteryx xanthostoma, Rolff & Siva-Jothy [49] present positive directional sexual selection gradients for immune function five to seven times higher than those we present as significant. These estimates are in accordance with sexual selection being a more potent force in nature than survival selection reported in recent quantitative reviews, although across 63 studies the median gradients for directional selection was 0.18 for mating success and 0.09 for survival (see [45,50,51]). This clearly concurs with the strength of the significant linear selection we found for both immune function and wing-spot size (table 1).
Stabilizing selection on immune function would result if the costs of maintaining and deploying the immune system were balanced by the benefits of pathogen and parasite resistance. Moreover, positive directional selection on immune function can be explained by either a high risk of attack by parasites that cause an encapsulation response or by condition dependence in immune function [49]. We suggest that the positive directional selection by birds on the immune system of their prey indicates that the benefits of deploying the immune system that are associated with a reduction in the likelihood of predation exceed the costs. We also highlight that these costs will include any costs of predator avoidance caused by handling and marking the insects. The benefits of the immune system will clearly be positively related to the likelihood of parasite or pathogen infection in the population. Calopterygids are often infected with parasitic gut protozoa (eugregarines [14,19]), and ectoparasitic mites [52], both of which were absent from the study population (J. Suhonen 2007, personal observation). However, our results may have reflected immigration from other parasite-laden populations or infection from unknown parasites and pathogens.
The mechanism(s) causing individuals with strong immune functions to also have reduced predation rates is unclear. However, it is unlikely to be an association with wing-spot size, because correlational selection between these two traits was non-significant. Previous studies on damselflies and other insects have shown condition dependence for immune defence [8,9,16,53]. We found no evidence for condition-dependent immune function in our study, although we cannot exclude the possibility for three reasons. (i) We estimated condition by measuring the effects of body mass independent of size before the immune system was challenged, and this may have changed during the study. (ii) Body mass per se may be less tightly correlated with condition than other traits, e.g. fat content [16], which was not measured as destructive sampling is required. (iii) There may not be a simple correlation between ‘condition’ and general immunity. In other words, encapsulation ability may not be condition-dependent, but other measures of immunity may be, although different aspects of immunity may be correlated with each other. The use of captive birds and damselflies for future studies would enable more detailed condition estimates and additional immune measurements to be taken. This would allow for the identification of traits that affect predation risk, especially any changes in damselfly behaviour that can be attributed to variation in the response of the immune system to a controlled challenge.
Our data are consistent with males that have stronger immune defences being most effective at avoiding birds (sensu [4]), possibly through being able to afford potentially costly behaviour or other traits associated with predator avoidance (e.g. [7]). Any cost of implementing a strong response to an immune challenge was thus unlikely to have been traded-off to a reduction in costly predator avoidance traits, at least in the short term. If this were so, we would expect stabilizing selection on immune function. Any traits associated with predator avoidance in the study animals are likely to be independent of both body size and wing length, both of which are associated with aerial manoeuvrability and subsequent predator avoidance in insects (e.g. [11,54]) but did not affect predation risk in our study. Alternatively, the wagtails may have been actively targeting potential prey displaying traits correlated with poor immune functions (see [55]). Immune system activation increases the likelihood and distances of dispersal in male C. virgo [21], so if it is also associated with increased general activity patterns this too could have led to an increased predation risk for individuals that exhibited high immune responses. Moreover, likely behavioural traits making individuals that displayed weak immune responses less likely to avoid, and/or more likely to be targeted by the birds may have been attributed at least in part by differences between individuals in their reactions to handling. This was clearly unavoidable because of the nature of the study, but further work examining other immune parameters with captive insects and predators could be used in the future to measure their effects.
In the same C. splendens population, immune-challenged males were less likely to become territorial than control males (26 versus 38%; [20]). Only 27 per cent of the males in this study were territorial, suggesting that territoriality rate was lower than normal but concurred with previous findings on immune-challenged males. This could explain the lack of any direct relationships between territoriality and immune response, and with predation risk. Unmanipulated territorial males tend to be fatter, and hence heavier and in better condition, than non-territorial males ([19]; reviewed by [56]). However, variance in territoriality in all males may have been reduced because of some reallocation of energy reserves to other unmeasured traits associated with predator avoidance after being immune-challenged. If part of their energy reserves were ‘sacrificed’ to ‘pay’ for predator avoidance [31], but not to such a level to affect survival, this would also explain why territorial males lived longer than non-territorial males independent of their condition. Only males of the highest ‘quality’ could have afforded to become territorial (see [16,57]).
The linear, independent selection on wing-spot size we detected contrasts with the correlational, stabilizing selection found in a Swedish C. splendens population also caused by wagtail predation ([11]; see §1). Svensson & Friberg [11] used slightly different wing-spot characters in their study, making direct comparison difficult, especially if different wing-spot characters correlate with immune function in different populations and/or with different components of immune function. We suggest that males with larger wing-spots were better at avoiding bird predation, were least likely to be directly targeted by the birds and/or were least affected by handling. Reduced targeting of individuals with large wing-spots has been suggested as counterintuitive because of their increased visibility [11], but there would be clear advantages to the birds to directly target males with small wing-spots if they were easier to catch. Males with small wing-spots are often forced into sub-prime habitats by more dominant intra-specific males with large wing-spots, or by the more aggressive males of their sympatric congener C. virgo [11,22,38]. However, we found no difference in wing-spot size between territorial and non-territorial males. Likewise, in contrast to previous studies on C. splendens [13,14] and other damselflies ([58,59]; see also [53]), we found no significant relationship between wing-spot size and immune response.
This is the first study to measure selection via a known agent, predation, on any aspect of immune function at the individual level in a wild animal population. Our data show that the selection was positively linear, and independent of other characteristics measured in the study, even though there was simultaneous positive linear selection for wing-spot size. Our study highlights the potential predation has for causing evolutionary change in the immune systems of their prey and the complexity of the relationship between immune function and secondary sexual traits (see e.g. [60–65]). Further research into the cues used by the birds to select prey, differences in predator and prey behaviour, prey morphology and prey immunology between populations, are therefore likely to be fruitful.
Acknowledgements
We thank the associate editor and the two reviewers for constructive comments that enabled us to improve the manuscript. We also thank Henna Aaltonen, Jaakko Ilvonen, Katariina Kangassalo, Kari Kaunisto, Janne Kumpulainen, Topias Laaksonen, Kalle Laitila, Pipsa Lappalainen, Urszula Marcinkowska, Lauri Rantanen, Eeva Rönnholm, Teijo Saikkonen, Anna Sipilä, Anssi Susilahti, Sini Tuomola and Heidi Viitaniemi for assistance in the laboratory and in the field. We thank Hanna Kokko, Erkki Korpimäki, Craig Primmer and Derek Roff for helpful comments on an early draft of the manuscript. Financial support was provided by the Academy of Finland (to M.J.R., J.H. and J.S.). D.W.D. is supported by a Leverhulme Trust Early Career Fellowship.
References
- 1.Darwin C. R. 1859. On the origin of species by natural selection or the preservation of favoured races in the struggle for life. London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- 2.Magnhagen C. 1991. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–185 10.1016/0169-5347(91)90210-O (doi:10.1016/0169-5347(91)90210-O) [DOI] [PubMed] [Google Scholar]
- 3.Hoey A. S., McCormick M. I. 2004. Selective predation for low body condition at the larval–juvenile transition of a coral reef fish. Oecologia 139, 23–29 10.1007/s00442-004-1489-3 (doi:10.1007/s00442-004-1489-3) [DOI] [PubMed] [Google Scholar]
- 4.Alzaga V., Vicente J., Villanua D., Acevedo P., Casas F., Gortazar C. 2008. Body condition and parasite intensity correlates with escape capacity in Iberian hares (Lepus granatensis). Behav. Ecol. Sociobiol. 62, 769–775 [Google Scholar]
- 5.Zuk M., Stoehr A. M. 2002. Immune defense and host life history. Am. Nat. 160, S9–S22 10.1086/342131 (doi:10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 6.Rigby M. C., Jokela J. 2000. Predator avoidance and immune defence: costs and trade-offs in snails. Proc. R. Soc. Lond. B 267, 171–176 10.1098/rspb.2000.0983 (doi:10.1098/rspb.2000.0983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kortet R., Rantala M. J., Hedrick A. 2007. Boldness in anti-predator behaviour and immune defence in field crickets. Evol. Ecol. Res. 9, 185–197 [Google Scholar]
- 8.Rantala M. J., Kortet R., Kotiaho J. S., Vainikka A., Suhonen J. 2003. Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor. Ecology 17, 534–540 [Google Scholar]
- 9.Yang S., Ruuhola T., Rantala M. J. 2007. Impacts of starvation on immune defense and other life history traits of an outbreaking geometrid, Epirrita autumnata: a possible ultimate trigger of the crash phase of population cycle. Ann. Zool. Fenn. 44, 89–96 [Google Scholar]
- 10.Folstad I., Karter A. J. 1992. Parasites, bright males and the immunocompetence handicap. Am. Nat. 139, 603–622 10.1086/285346 (doi:10.1086/285346) [DOI] [Google Scholar]
- 11.Svensson E. I., Friberg M. 2007. Selective predation on wing morphology in sympatric damselflies. Am. Nat. 170, 101–112 10.1086/518181 (doi:10.1086/518181) [DOI] [PubMed] [Google Scholar]
- 12.Siva-Jothy M. T. 1999. Male wing pigmentation may affect reproductive success via female choice in a calopterygid damselfly (Zygoptera). Behaviour 136, 1365–1377 10.1163/156853999500776 (doi:10.1163/156853999500776) [DOI] [Google Scholar]
- 13.Rantala M. J., Koskimäki J., Taskinen J., Tynkkynen K., Suhonen J. 2000. Immunocompetence, developmental stability and wingspot size in the damselfly Calopteryx splendens. Proc. R. Soc. Lond. B 267, 2453–2457 10.1098/rspb.2000.1305 (doi:10.1098/rspb.2000.1305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siva-Jothy M. T. 2000. A mechanistic link between parasite resistance and expression of a sexually selected trait in a damselfly. Proc. R. Soc. Lond. B 267, 2523–2527 10.1098/rspb.2000.1315 (doi:10.1098/rspb.2000.1315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper R. E., Tsubaki Y., Siva-Jothy M. Y. 1999. Expression of a costly secondary trait is correlated with age and condition in a damselfly with two male morphs. Physiol. Entomol. 24, 364–369 10.1046/j.1365-3032.1999.00152.x (doi:10.1046/j.1365-3032.1999.00152.x) [DOI] [Google Scholar]
- 16.Koskimäki J., Rantala M. J., Taskinen J., Tynkkynen K., Suhonen J. 2004. Immunocompetance and resource holding potential in the damselfly, Calyopteryx virgo. Behav. Ecol. 15, 169–173 10.1093/beheco/arg088 (doi:10.1093/beheco/arg088) [DOI] [Google Scholar]
- 17.Contreras-Garduño J., Buzatto B. A., Serrano-Meneses M. A., Nájera-Cordero K., Córdoba-Aguilar A. 2008. The size of the red wing-spot of the American rubyspot as a heightened condition-dependent ornament. Behav. Ecol 19, 724–732 10.1093/beheco/arn026 (doi:10.1093/beheco/arn026) [DOI] [Google Scholar]
- 18.Askew R. R. 1988. The dragonflies of Europe. Colchester, UK: Harley Books [Google Scholar]
- 19.Siva-Jothy M. T., Plaistow S. J. 1999. A fitness cost of eugregarine parasitism in a damselfly. Ecol. Entomol. 25, 465–470 [Google Scholar]
- 20.Rantala M. J., Honkavaara J., Suhonen J. 2010. Immune system activation interacts with territory-holding potential and increases predation of the damselfly Calopteryx splendens by birds. Oecologia 163, 825–832 10.1007/s00442-010-1582-8 (doi:10.1007/s00442-010-1582-8) [DOI] [PubMed] [Google Scholar]
- 21.Suhonen J., Honkavaara J., Rantala M. J. 2010. Activation of the immune system promotes insect dispersal in the wild. Oecologia 162, 541–547 10.1007/s00442-009-1470-2 (doi:10.1007/s00442-009-1470-2) [DOI] [PubMed] [Google Scholar]
- 22.Tynkkynen K., Rantala M. J., Suhonen J. 2004. Interspecific aggression and character displacement in the damselfly Calopteryx splendens. J. Evol. Biol. 17, 759–768 10.1111/j.1420-9101.2004.00733.x (doi:10.1111/j.1420-9101.2004.00733.x) [DOI] [PubMed] [Google Scholar]
- 23.Honkavaara J., Rantala M. J., Suhonen J. 2009. Mating status, immune defence, and multi-parasite burden in the damselfly Coenagrion amatum. Entomol. Exp. Appl. 132, 165–171 10.1111/j.1570-7458.2009.00877.x (doi:10.1111/j.1570-7458.2009.00877.x) [DOI] [Google Scholar]
- 24.Forbes M. R., Muma K. E., Smith B. P. 1999. Parasitism of Sympetrum dragonflies by Arrenurus planus mites: maintenance of resistance particular to one species. Int. J. Parasitol. 29, 991–999 10.1016/S0020-7519(99)00061-2 (doi:10.1016/S0020-7519(99)00061-2) [DOI] [PubMed] [Google Scholar]
- 25.Yourth C. P., Forbes M. R., Baker R. L. 2002. Sex differences in melanotic encapsulation responses (immunocompetence) in the damselfly Lestes forcipatus Rambur. Can. J. Zool. 80, 1578–1583 10.1139/z02-159 (doi:10.1139/z02-159) [DOI] [Google Scholar]
- 26.Gorman M. J., Cornel A. J., Collins F. H., Paskewitz S. M. 1996. A shared genetic mechanism for melanotic encapsulation of CM-sephadex beads and the malaria parasite, Plasmodium cynomolgi B, in the mosquito. Anophiles gambiae. Exp. Parasitol. 84, 380–386 10.1006/expr.1996.0126 (doi:10.1006/expr.1996.0126) [DOI] [PubMed] [Google Scholar]
- 27.Smilanich A. M., Dyer L. E., Gentry G. L. 2009. The insect immune response and other putative defenses as effective predictors of parasitism. Ecology 90, 1434–1440 10.1890/08-1906.1 (doi:10.1890/08-1906.1) [DOI] [PubMed] [Google Scholar]
- 28.Rantala M. J., Roff D. A. 2007. Inbreeding and extreme outbreeding causes sex differences in immune defence and life history traits in Epirrita autumnata. Heredity 98, 329–336 10.1038/sj.hdy.6800945 (doi:10.1038/sj.hdy.6800945) [DOI] [PubMed] [Google Scholar]
- 29.Ryder J. J. 2007. Temporal dynamics of the encapsulation response towards a synthetic immune challenge in Acheta domesticus. Physiol. Entomol. 32, 240–245 10.1111/j.1365-3032.2007.00572.x (doi:10.1111/j.1365-3032.2007.00572.x) [DOI] [Google Scholar]
- 30.Rantala M. J., Roff D. A. 2006. Analysis of the importance of genotypic variation, metabolic rate, morphology, sex and development time on immune function in the cricket, Gryllus firmus.. J. Evol. Biol. 19, 834–843 10.1111/j.1420-9101.2005.01048.x (doi:10.1111/j.1420-9101.2005.01048.x) [DOI] [PubMed] [Google Scholar]
- 31.Toivanen T., Rantala M. J., Suhonen J. 2009. Influence of alternative mating tactics on predation risk in the damselfly Calopteryx virgo. Can. J. Zool. 87, 684–688 10.1139/Z09-055 (doi:10.1139/Z09-055) [DOI] [Google Scholar]
- 32.Ward L., Mill P. J. 2006. Diel activity patterns in the adult banded demoiselle, Calopteryx splendens (Harris), and the effect of weather variables. J. Br. Dragonfly Soc. 22, 58–63 [Google Scholar]
- 33.Tynkkynen K., Kotiaho J. S., Luojumäki M., Suhonen J. 2005. Interspecific aggression causes negative selection on sexual characters. Evolution 59, 1838–1843 [PubMed] [Google Scholar]
- 34.Grether G. F. 1996. Sexual selection and survival selection on wing coloration and body size in the rubyspot damselfly Hetaerina Americana. Evolution 50, 1939–1948 10.2307/2410752 (doi:10.2307/2410752) [DOI] [PubMed] [Google Scholar]
- 35.Forsyth A., Montgomerie D. R. 1987. Alternative reproductive tactics in the territorial damselfly Calopteryx maculate: sneaking by older males. Behav. Ecol. Soc. 21, 73–81 10.1007/BF02395434 (doi:10.1007/BF02395434) [DOI] [Google Scholar]
- 36.Plaistow S., Siva-Jothy M. T. 1996. Energetic constraints and male mate-securing tactics in the damselfly Calopteryx splendens xanthostoma (Charpentier). Proc. R. Soc. Lond. B 263, 1233–1238 10.1098/rspb.1996.0181 (doi:10.1098/rspb.1996.0181) [DOI] [Google Scholar]
- 37.Rainhani G., Serrano-Meneses M. A., Córdoba-Aguilar A. 2008. Male mating tactics in the American rubyspot damselfly: territoriality, nonterritoriality and switching behaviour. Anim. Behav. 75, 1851–1860 [Google Scholar]
- 38.Tynkkynen K., Kotiaho J. S., Luojumäki M., Suhonen J. 2006. Interspecific territoriality in Calopteryx damselflies: the role of secondary sexual characters. Anim. Behav. 71, 299–306 10.1016/j.anbehav.2005.03.042 (doi:10.1016/j.anbehav.2005.03.042) [DOI] [Google Scholar]
- 39.Lande R., Arnold S. J. 1983. Measuring selection on correlated characters. Evolution 37, 1210–1226 10.2307/2408842 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 40.Janzen F. J., Stern H. S. 1998. Logistic regression for empirical studies of multivariate selection. Evolution 52, 1564–1571 10.2307/2411330 (doi:10.2307/2411330) [DOI] [PubMed] [Google Scholar]
- 41.Stinchcombe J. R., Agrawal A. F., Hohenloe P. A., Arnold S. A. 2008. Estimating nonlinear selection gradients using quadratic regression: double or nothing? Evolution 62, 2435–2440 10.1111/j.1558-5646.2008.00449.x (doi:10.1111/j.1558-5646.2008.00449.x) [DOI] [PubMed] [Google Scholar]
- 42.Phillips P. C., Arnold S. J. 1989. Visualizing multivariate selection. Evolution 44, 1149–1222 [DOI] [PubMed] [Google Scholar]
- 43.Blows M. W., Brooks R. 2003. Measuring nonlinear selection. Am. Nat. 162, 815–820 10.1086/378905 (doi:10.1086/378905) [DOI] [PubMed] [Google Scholar]
- 44.Blows M. W. 2007. A tale of two matrices: multivariate approaches in evolutionary biology. J. Evol. Biol. 20, 1–8 10.1111/j.1420-9101.2006.01164.x (doi:10.1111/j.1420-9101.2006.01164.x) [DOI] [PubMed] [Google Scholar]
- 45.Kingsolver J. G., Hoekstra H. E., Hoekstra J. M., Berrigan D., Vignieri S. N., Hill C. E., Hoang A., Gibert P., Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261 10.1086/319193 (doi:10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S. 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 15, 1044–1045 10.1093/beheco/arh107 (doi:10.1093/beheco/arh107) [DOI] [Google Scholar]
- 47.Brodie E. D., McGlothlin J. W. 2007. A cautionary tale of two matrices: the duality of multivariate abstraction. J. Evol. Biol. 20, 9–14 10.1111/j.1420-9101.2006.01219.x (doi:10.1111/j.1420-9101.2006.01219.x) [DOI] [PubMed] [Google Scholar]
- 48.Råberg L., Stjernman M. 2003. Natural selection on immune responsiveness in blue tits, Parus caeuleus. Evolution 57, 1210–1226 [DOI] [PubMed] [Google Scholar]
- 49.Rolff J., Siva-Jothy M. T. 2004. Selection on insect immunity in the wild. Proc. R. Soc. Lond. B 271, 2157–2160 10.1098/rspb.2004.2859 (doi:10.1098/rspb.2004.2859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoekstra H. E., Hoekstra J. M., Berrigaan D., Vignieri S. N., Hoang A., Hill C. E., Beerli P., Kingsolver J. G. 2001. Strength and tempo of directional selection in the wild. Proc. Natl Acad. Sci. USA 98, 9157–9160 10.1073/pnas.161281098 (doi:10.1073/pnas.161281098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kingsolver J. G., Pfenning D. W. 2007. Patterns and power of phenotypic selection in nature. Bioscience 57, 561–572 10.1641/B570706 (doi:10.1641/B570706) [DOI] [Google Scholar]
- 52.Åbro A. 1982. The effect of parasitic water mite larvae (Arrenurus spp.) on zygopteran imagoes (Odonata). J. Invert. Pathol. 39, 373–381 [Google Scholar]
- 53.Córdoba-Aguilar A., Jiménez-Cortéz J. G., Lanz-Mendoza H. 2009. Seasonal variation in ornament expression, body size, energetic reserves, and survival in males of a territorial insect. Ecol. Entomol 34, 228–239 10.1111/j.1365-2311.2008.01061.x (doi:10.1111/j.1365-2311.2008.01061.x) [DOI] [Google Scholar]
- 54.Neems R. M., Lazarus J., McLachlan A. J. 1998. Lifetime reproductive success in a swarming midge: trade-offs and stabilizing selection for male body size. Behav. Ecol. 9, 279–286 10.1093/beheco/9.3.279 (doi:10.1093/beheco/9.3.279) [DOI] [Google Scholar]
- 55.Lafferty K. D., Morris A. K. 1996. Altered behavior of parasitized killfish increases susceptibility to predation by bird final hosts. Ecology 77, 1390–1397 10.2307/2265536 (doi:10.2307/2265536) [DOI] [Google Scholar]
- 56.Suhonen J., Rantala M. J., Honkavaara J. 2008. Territoriality in odonates. In Dragonflies and damselflies: model organisms for ecological and evolutionary research (ed. Córdoba-Aguilar A.), pp. 203–217 Oxford, UK: Oxford University Press [Google Scholar]
- 57.Contreras-Garduno J., Cordoba-Aguilar A., Lanz-Mendoza H., Cordero-Rivera A. 2009. Territorial behaviour and immunity are mediated by juvenile hormone: the physiological basis of honest signalling? Funct. Ecol. 23, 157–163 10.1111/j.1365-2435.2008.01485.x (doi:10.1111/j.1365-2435.2008.01485.x) [DOI] [Google Scholar]
- 58.Contreras-Garduño J., Canales-Lazcano J., Córdoba-Aguilar A. 2006. Wing pigmentation, immune ability and fat researves in males of the rubyspot damselfly, Hetaerina americana. J. Ethol. 24, 165–173 [Google Scholar]
- 59.Contreras-Garduño J., Lanz-Mendoza H., Córdoba-Aguilar A. 2007. The expression of a sexually selected trait correlates with different immune defence components and survival in males of the American rubyspot. J. Insect Physiol. 53, 612–621 10.1016/j.jinsphys.2007.03.003 (doi:10.1016/j.jinsphys.2007.03.003) [DOI] [PubMed] [Google Scholar]
- 60.Ryder J. J., Siva-Jothy M. T. 2000. Male calling song provides reliable information about immune function in a cricket. Proc. R. Soc. Lond. B 267, 1171–1175 10.1098/rspb.2000.1125 (doi:10.1098/rspb.2000.1125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rantala M. J., Jokinen I., Kortet R., Vainikka A., Suhonen J. 2002. Do pheromones reveal immunocompetence? Proc. R. Soc. Lond. B 269, 1681–1685 10.1098/rspb.2002.2056 (doi:10.1098/rspb.2002.2056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rantala M. J., Kortet R. 2003. Courtship song and immune function in the field cricket Gryllus bimaculatus? Biol. J. Linn. Soc. 79, 503–510 10.1046/j.1095-8312.2003.00202.x (doi:10.1046/j.1095-8312.2003.00202.x) [DOI] [Google Scholar]
- 63.Ahtiainen J. J., Alatalo R. V., Kortet R., Rantala M. J. 2004. Sexual advertisement and immune function in an arachnid species (Lycosidae). Behav. Ecol. 15, 602–606 10.1093/beheco/arh062 (doi:10.1093/beheco/arh062) [DOI] [Google Scholar]
- 64.Simmons L. W., Zuk M., Rotenberry J. T. 2005. Immune function reflected in calling song characteristics in a natural population of the cricket Teleogryllus commodus. Anim. Behav. 69, 1235–1241 10.1016/j.anbehav.2004.09.011 (doi:10.1016/j.anbehav.2004.09.011) [DOI] [Google Scholar]
- 65.Rantala M. J., Roff D. A., Rantala L. M. 2007. Forceps size and immune function in the European earwig Forficula auricularia. Biol. J. Linn. Soc. 90, 509–516 10.1111/j.1095-8312.2007.00741.x (doi:10.1111/j.1095-8312.2007.00741.x) [DOI] [Google Scholar]


