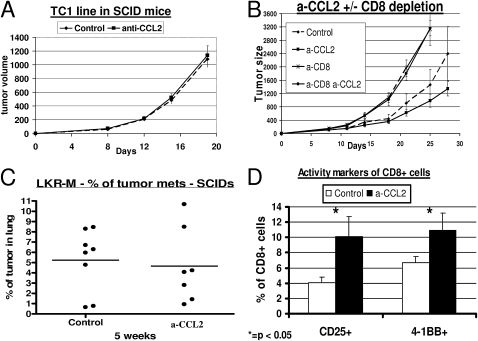
Figure 4.
Effect of CCL2 blockade on thoracic malignancies is dependent on intratumoral CD8+ T cells. (A) CB-17 severe combined immunodeficient (SCID) mice (n = 5–6 for each group) bearing 200-mm3 TC1 tumors were treated with saline (Control) or intraperitoneal α-CCL2 mAb (a-CCL2). No difference in the pattern of tumor growth was evident between the two groups. (B) Immunocompetent C57bL/6 mice (n = 5–6 for each group) bearing large TC1 tumors were treated with: (1) saline (diamonds, Control); (2) intraperitoneal α-CCL2 mAb (a-CCL2) (squares, a-CCL2); (3) saline, and injected with 300 μg of an anti-CD8 mAb intraperitoneal twice weekly (crosses, a-CD8); and (4) α-CCL2 mAB and depletion of CD8+ cells (circles, a-CD8 a-CCL2). Control and a-CCL2 groups were treated with an intraperitoneal control IgG antibody. The effect of CCL2 blockade on tumor growth disappeared completely in mice depleted of CD8+ T cells. (C) SCID mice (n = 7–8 for each group) were injected on the right flank with the NSCLC cell line LKR-M. After flank tumors reached an average size of 200–250 mm3, mice were treated with either saline (Control) or intraperitoneal α-CCL2/CCL12 mAb twice weekly (a-CCL2). After 5 weeks of treatment, lungs were evaluated for metastases. The percentage of lung occupied by metastases did not differ between the two groups. Each dot represents one mouse. (D) Percentages of intratumoral CD8+ T cells expressing the two activation markers, CD25 (left) and 4-1BB (right). The percentage of activated cells out of all CD8+ T cells was doubled in mice treated with a-CCL2 mAb (*P < 0.05).