Figure 1.
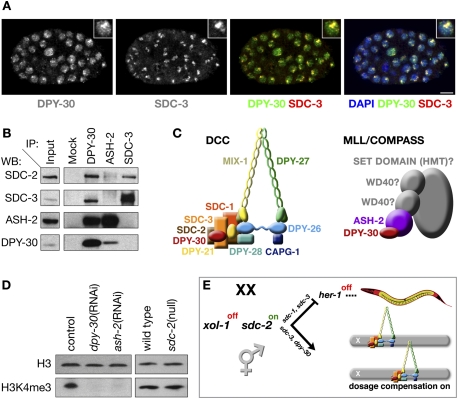
DPY-30 participates in two gene regulatory complexes: the DCC and MLL/COMPASS. (A) DPY-30 exhibits both diffuse nuclear localization and enrichment on X colocalized with DCC subunits. Confocal images of an XX embryo costained with 4,6-diamidino-2-phenylindole (DAPI, blue), and antibodies against DPY-30 (green in merge) and SDC-3 (red in merge). DPY-30 and SDC-3 colocalize (yellow). The diffuse nuclear localization of DPY-30 supports a function beyond DC. Inset is an enlarged nucleus. Bar, 5 μm. (B) DPY-30 interacts with subunits of the DCC and the MLL/COMPASS complex. Immunoprecipitation (IP) and Western blot (WB) analysis using embryo extracts confirm association of DPY-30 with the DCC subunits SDC-2 and SDC-3 as well as the MLL/COMPASS subunit ASH-2. Immunoprecipitation of ASH-2 fails to recover SDC-3 and very weakly recovers SDC-2, indicating that DPY-30 likely functions in two distinct complexes. (C) Schematics of the C. elegans DCC and C. elegans MLL/COMPASS complexes with known subunits identified (in color). DPY-30 (red) is shared between both complexes. (D) C. elegans DPY-30 and ASH-2 are required for H3K4me3, consistent with their participation in MLL/COMPASS. Shown are Western blots of either RNAi empty vector control (L4440), dpy-30(RNAi), ash-2(RNAi), wild-type, or sdc-2 (null) mutant embryos blotted with antibodies to histone H3 or H3K4me3. H3K4me3 is undetectable in embryos depleted of DPY-30 or ASH-2, while H3 levels are unaffected. H3K4me3 levels are not reduced in sdc-2 mutant embryos, confirming that participation of DPY-30 in MLL/COMPASS, and not the DCC, is responsible for H3K4me3, and that disruption of DC does not affect H3K4me3. (E) Assembly of the DCC onto X is controlled by a genetic pathway that regulates both sex determination and DC in which repression of xol-1 activity in XX animals permits activation of the XX-specific gene sdc-2. sdc-2 turns on the hermaphrodite pathway of sexual differentiation in collaboration with sdc-1 and sdc-3 by repressing the male sex determination gene her-1, and sdc-2 triggers loading of the DCC onto X chromosomes in concert with sdc-3 and dpy-30 (Meyer 2010).