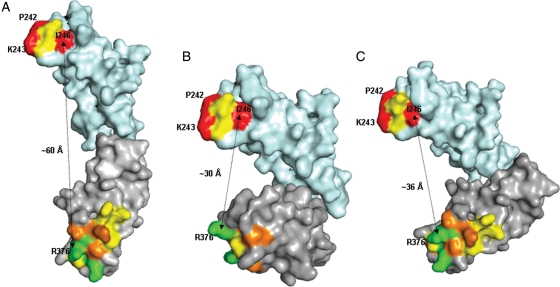
Fig. 6.
Alternative arrangements of C2 (cyan) and C3 (grey) domains of CD22 obtained by superposition of the models of these domains on (A), a model of carcinoembryonic antigen (domains 3 and 4; 1e07.pdb), (B) crystal structure of human Fcγ-receptor (2fcb.pdb) and (C) crystal structure of leukocyte Ig-like receptor A5 (2d3v.pdb). Residues mutated to alanine are coloured according to the percentage of wild-type binding to CAT-8015, upon alanine substitution (red, 0–20% residual binding; orange, 20–50% residual binding; yellow, 50–80% residual binding; green, 80–100% residual binding). Approximate distance between C-α atoms of I246 in the C2 domain and R376 in the C3 domain are shown as an indication of the proximity of the epitope clusters (cluster 1 at the top and cluster 2 at the bottom).