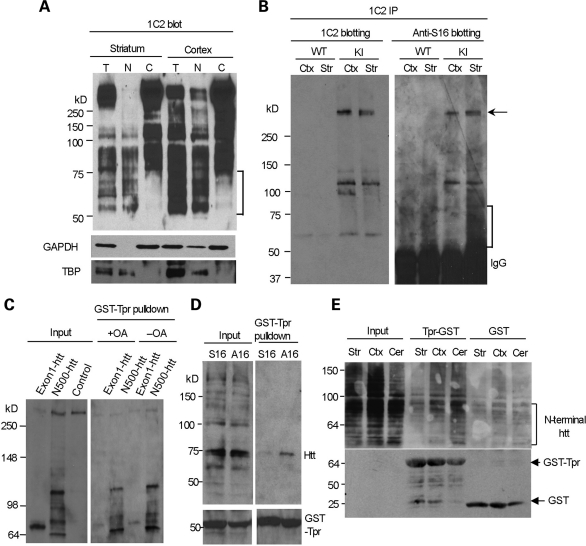
Figure 7.
N-terminal mutant htt with phosphorylated S16 is enriched in the nuclear fraction of striatal neurons and shows decreased binding to Tpr. (A) 1C2 western blotting revealing that N-terminal mutant htt fragments <75 kDa (marked by the bracket) are present only in the total and nuclear fractions, but not in the cytosolic fractions, of the mouse striatum and cortex. The same blot was also probed with antibodies to the cytoplasmic protein GAPDH and the nuclear protein TBP. (B) Mutant htt in CAG140 KI mouse striatum and cortex was immunoprecipitated by 1C2. Western blots with anti-S16 and 1C2 showed more abundant N-terminal fragments (marked by the bracket) in the striatum that were labeled by anti-S16. Densitometry analysis (lower panel) of the relative percentage of phosphorylated full-length htt by measuring the intensity of htt labeled by anti-S16 relative to htt labeled by 1C2. (C) HEK293 cells were cotransfected with GST-Tpr and exon1-150Q or N500-htt-140Q htt. More N500-htt-140Q and its degraded products than exon1-150Q were precipitated by GST-Tpr. Note that very little full-length normal htt (arrow) was precipitated and that increased phosphorylation by OA treatment reduces the interaction of N-terminal htt with GST-Tpr. (D) HEK293 cells were cotransfected with GST-Tpr (bracket) and N208-143Q containing S16 or A16. A GST pulldown followed by western blotting with mEM48 showed that phosphorylation elimination by A16 substitution increased the association of htt with Tpr. (E) Striatal, cortical and cerebellar lysates were prepared from CAG140 KI mice and incubated with GST-Tpr or GST alone. Western blotting of the GST pulldown samples with 1C2 showed that the binding of N-terminal htt fragments (marked by the bracket) to Tpr was reduced in the striatum. The blot was also probed with an antibody to GST to reveal GST and GST-Tpr used for the precipitation of mouse brain mutant htt.