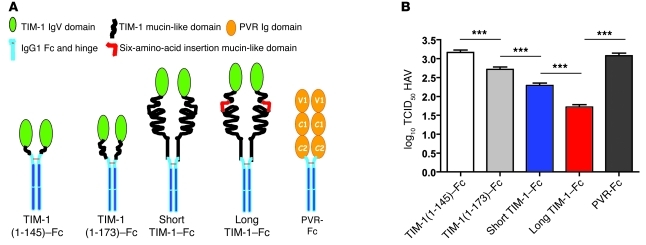
Figure 1. TIM1 polymorphisms affect HAV binding and uncoating.
(A) The TIM-1–Fc fusion protein constructs used. Soluble TIM-1 fusion protein constructs containing the TIM-1 IgV domain and different lengths of the mucin domain were generated. The constructs contained the TIM-1 IgV domain and (a) the first 19 residues of the mucin domain (TIM-1[1–145]–Fc); (b) the first 42 residues of the mucin domain without the 157insMTTTVP insertion polymorphism (TIM-1[1–173]–Fc); (c) the entire mucin allele without the 157insMTTTVP insertion polymorphism (Short TIM-1–Fc); and (d) the entire mucin allele containing the 6-amino-acid insertion polymorphism (157insMTTTVP) (Long TIM-1–Fc). A Fc fusion protein containing the extracellular domain of the poliovirus receptor (PVR-Fc) with 3 Ig-like domains (V1, C1, and C2) was used as a negative control. (B) The long form of TIM-1 binds HAV more effectively than the shorter form. HAV was treated with the purified soluble fusion proteins described in A, which resulted in HAV neutralization. Residual HAV infectivity was determined by end-point ELISA titration assay in African green monkey kidney cells, and expressed as log10 of TCID50 ± SD (***P < 0.001 [ANOVA]). Lower infectivity indicates more effective binding of HAV to the designated TIM-1 form.