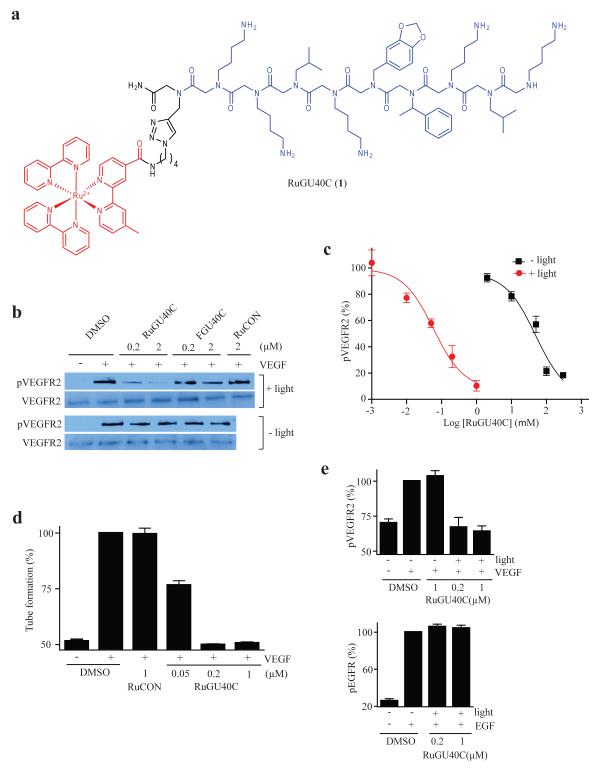
Figure 1.
Visible light-triggered inactivation of the Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) by a ruthenium-peptoid conjugate. (a) Chemical structure of RuGU40C. The modified Ru(II)(bpy)32+ complex and the GU40C peptoid are shown in red and blue, respectively. (b) Western blots showing the level of phospho-VEGFR2 (the active form of the receptor) and total VEGFR2 after receptor-expressing cells (PAE/KDR) were incubated under the conditions indicated. The duration of irradiation was 10 minutes. FGU40C = fluorescein-conjugated GU40C (see Supplementary Fig. 2). RuCON = a Ru(II)(bpy)32+-conjugated control peptoid that does not bind VEGFR2 (see Supplementary Fig. 2). (c) Dose-dependence of the inhibition of autophosphorylation of VEGFR2 by RuGU40C with or without irradiation. (d) Effect of ruthenium-peptoid conjugates on the VEGF-induced formation of tubes by human umbilical vascular endothelial cells (HUVECs). HUVECs on Matrigel-coated plates were incubated under the conditions indicated and irradiated (10 min). 16hr after the addition of VEGF, degree of tube formation was evaluated by quantitative analysis (AngioQuant software) of images obtained using a light microscope (see Fig S3 for representative images). (e) Analysis of the specificity of RuGU40C-mediated inhibition of VEGFR2. The effect of the ruthenium-peptoid conjugate on hormone-mediated autophosphorylation (activation) of VEGFR2 or EGFR was examined by western blot in the presence or absence of irradiation (10 min) in cells that express both receptors (H441) and evaluated by quantitative analysis (Image J). Note that there is a basal level of phosph-VEGFR2 present even in the absence of VEGF treatment.