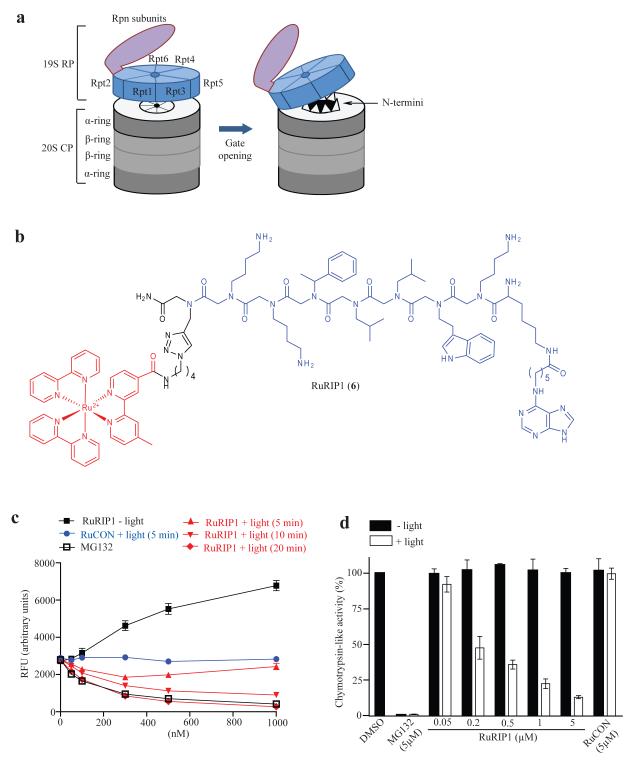
Figure 3.
Visible light-triggered inactivation of the 26S proteasome by a ruthenium-peptoid conjugate. (a) Illustration of the 26S proteasome and gate opening of the 20S proteasome. (b) Chemical structure of RuRIP1. The modified Ru(II) (bpy) 2+3 complex and RIP1 peptoid are shown in red and blue, respectively. (c) Chymotrypsin-like peptidase activity of purified, yeast 26S proteasome was measured in the presence of RuRIP1 with or without irradiation by monitoring the cleavage of fluorogenic substrate, Suc-LLVY-AMC. (d) The effect of RuRIP1 on chymotrypsin-like activity of the 26S proteasome in HeLa cells with or without irradiation (30 min) was assessed by measuring luminescence generated by substrate (Suc-LLVY-aminoluciferin) cleavage.