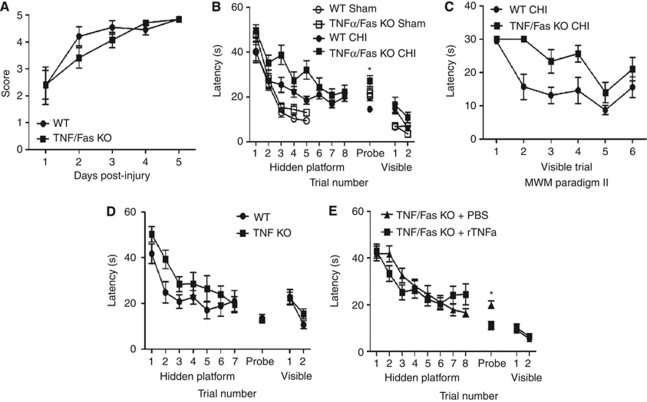
Figure 4.
Motor and Morris water maze (MWM) deficits (assessed at days 1 to 4 after CHI) in TNFα/Fas KO and WT mice after closed head injury (CHI). (A) After CHI, TNFα/Fas KO and WT animals had transient motor deficits versus sham-injured WT mice (P<0.01 compared with sham-injured mice in Figure 4). TNFα/Fas KO and WT mice did not differ in postinjury wire-grip performance after CHI (P=0.36; n=15 per group). (B) Sham-injured and injured TNFα/Fas KO and WT mice improved their MWM performance in hidden platform trials over time (P<0.0001 time effect for both groups). Sham-injured WT and TNFα/Fas KO animals (n=12 per group) did not differ in hidden platform, visible platform, or probe trial performance. Injured TNFα/Fas KO mice had worse hidden platform trial latencies compared with injured WT (P=0.036, group effect; n=15 per group); however, injured TNFα/Fas KO mice performed significantly better than injured WT in probe trials (*P=0.0001). (C) Injured TNFα/Fas KO mice preformed significantly worse than WT in visible platform trials in MWM paradigm II, suggesting a nonspatial, procedural deficit (P=0.0015, group effect; n=7 per group). (D) Compared with injured WT, injured TNFα KO mice had similar performance in hidden platform trials (P=0.09, group effect; n=12 per group) and probe trials (P=0.73). (E) TNFα/Fas KO mice were injected with recombinant TNFα or PBS and then subjected to CHI. TNF/Fas KO mice injected with TNFα had worse probe trial performance compared with TNFα/Fas KO mice administered PBS (*P=0.0025; n=13 per group). There was no difference between groups in hidden platform or visible platform trials (P>0.60 for hidden and visible platform trials). CHI, closed head injury; KO, knockout; PBS, phosphate-buffered saline; TNFα, tumor necrosis factor-α; WT, wild type.