Abstract
One of the major virulence factors of the malaria causing parasite is the Plasmodium falciparum encoded erythrocyte membrane protein 1 (PfEMP1). It is translocated to It the membrane of infected erythrocytes and expressed from approximately 60 var genes in a mutually exclusive manner. Switching of var genes allows the parasite to alter functional and antigenic properties of infected erythrocytes, to escape the immune defense and to establish chronic infections. We have developed an efficient method for isolating VAR genes from telomeric and other genome locations by adapting transformation-associated recombination (TAR) cloning, which can then be analyzed and sequenced. For this purpose, three plasmids each containing a homologous sequence representing the upstream regions of the group A, B, and C var genes and a sequence homologous to the conserved acidic terminal segment (ATS) of var genes were generated. Co-transfection with P. falciparum strain ITG2F6 genomic DNA in yeast cells yielded 200 TAR clones. The relative frequencies of clones from each group were not biased. Clones were screened by PCR, as well as Southern blotting, which revealed clones missed by PCR due to sequence mismatches with the primers. Selected clones were transformed into E. coli and further analyzed by RFLP and end sequencing. Physical analysis of 36 clones revealed 27 distinct types potentially representing 50% of the var gene repertoire. Three clones were selected for sequencing and assembled into single var gene containing contigs. This study demonstrates that it is possible to rapidly obtain the repertoire of var genes from P. falciparum within a single set of cloning experiments. This technique can be applied to individual isolates which will provide a detailed picture of the diversity of var genes in the field. This is a powerful tool to overcome the obstacles with cloning and assembly of multi-gene families by simultaneously cloning each member.
Introduction
Malaria tropica is caused by the apicomplexan parasite Plasmodium falciparum and accounts for approximately 500 million cases with a death toll of 800'000 annually [1]. Nearly all pathology is caused by the intimate interaction between the infected erythrocyte and the host's capillary endothelium, which is conferred by a parasite-derived molecule in the membrane of the erythrocyte, termed erythrocyte membrane protein 1 (PfEMP1). It is considered to be the major virulence factor of P. falciparum [2], [3]. PfEMP1 shows antigenic variation and is encoded by about 60 different var genes per haploid genome. The expression of a PfEMP1 variant is mutually exclusive [4], [5] and the protein is a major target of the adaptive immune response. It is believed that protection against clinical disease depends on the number of different PfEMP1 variants previously seen by the immune system [6], [7]. A significant number of these proteins are able to bind to different endothelial receptors such as CD36 or ICAM-1, leading to sequestration of infected red blood cells from the blood stream, thus avoiding clearance by the immune system and the spleen [8]. Switching expression of var genes is often accompanied by differences in the binding phenotype, allowing the parasite to escape the host's immune response.
var genes vary in their size from 6 to 13 kb [9]. They are flanked by a large upstream promoter region that is considered to play an important role in the regulation of var genes [10], [11]. The P. falciparum sequencing project revealed the complete set of var genes of the 3D7 strain. They can be grouped into group A, B and C var genes according to their 5′ upstream regions [12], [13]. Group A and B var genes are located almost exclusively at telomere ends, where upsA var genes are transcribed towards the telomere and upsB var genes towards the centromere. In contrast, upsC var genes are located in central chromosomal regions [8]. Such distribution of group A, B, and C var genes has also been observed in parasite samples from naturally infected patients, however, sequence diversity appears to be vast, perhaps unlimited, with thousands of different Duffy-binding-like (DBL) domains already identified in parasite field isolates [14]–[16].
var genes possess a two exon structure with the first exon between 3.5 to 9 kb in size [12]. Exon 1 encodes the highly variable region that is expressed on the surface of infected red blood cells as well as a transmembrane domain. Each var gene comprises a distinct and unique set of domains which most probably have evolved through recombination from a common ancestor. The most conserved domain is the DBL1alpha domain with identities of up to 52% between different var genes [17]. Following an AT-rich intron, exon 2 encodes the more conserved amino terminal segment (ATS) that is located in the intracellular part of the infected red blood cell.
Sequencing downstream of the DBL1alpha domain poses difficulties because of the high AT richness, long homopolymeric sequence stretches, repeat units of various sizes and the fact that var genes evolve by frequent recombination. Both Sanger sequencing and next generation sequencing of whole genomes fail to unequivocally determine sequences of full-length var genes due to difficulties in the assembly of var genes de novo or onto existing scaffolds. To overcome these limitations we applied a technique that could specifically generate clones containing single var gene units. This information could provide important insights into the pathology of malaria. So far, only a very small number of var genes have been associated with distinct clinical presentations, such as the var2csa gene (PPL_003c) encoding a PfEMP1 variant that binds to chondroitin sulfate A and is responsible for pregnancy-associated malaria [17]. Few studies were able to associate upstream regions of group A, B, and C var genes as well as the DBL1alpha domain in malaria infected children from endemic areas with clinical disease presentation [3], [15], [16], [18], [19], [20], [21]. However, analysis of complete var genes from patient samples might result in a different or more complete picture of parasite-host interactions involving PfEMP1.
Transformation associated recombination (TAR) cloning has been successfully used for cloning of telomeric ends in various organisms [22], for cloning of centromere regions [23], [24], for gap closure of difficult sequence stretches [25], and for selectively cloning vsg–expression sites from Trypanosoma brucei [26], [27]. We applied a modified protocol of transformation-associated recombination [28], [29] to isolate individual var genes from P. falciparum strain ITG2F6. The Brazilian strain IT4/25/4, the parental strain of ITG2F6, has been partially sequenced and its var genes have been compared to those of 3D7 and HB3 [30]. This allows comparison of isolated var gene sequences of the ITG2F6 strain with IT4/25/4 sequences and facilitates sequence analysis.
TAR cloning offers several advantages over traditional cloning methods when genomic regions are difficult to deal with [29] as demonstrated with the cloning and characterization of bloodstream form expression sites from T. brucei [26], [27]. In the case of var genes, the alternative to TAR would be whole genome sequencing projects or the screening of random insert libraries rather than efficient targeted library construction using TAR. Here, we present the construction of a var clone library containing about 200 clones that were obtained in experiments using three different TAR cloning vectors specific for upsA, upsB and upsC var genes. We demonstrate the feasibility of analyzing these clones by PCR colony screening and Southern blotting. A selected number of var clones were transformed into E. coli, allowing plasmid preparations of high concentrations, for restriction fragment length polymorphism (RFLP) analyses or sequencing. Moreover, we used deep sequencing on three isolated var clones and were able to assemble them de novo.
TAR cloning can be used as a rapid and efficient method for the isolation of var genes of different P. falciparum strains. Moreover, utilization of this technique on field samples of infected patients will greatly support studies aiming to associate certain var gene types (present or expressed) with particular clinical presentations. Combined with long range PCR, this technique will provide a means to study the regulation and dynamics of var genes in naturally infected individuals and the associated functional significance of var gene expression that currently is an impossible task. Furthermore, it will provide data on the diversity of the entire full-length var gene repertoire.
Results
Construction of TAR library containing var genes from P. falciparum strain ITG2F6
In order to isolate the repertoire of single var genes from the P. falciparum strain ITG2F6, a modified TAR cloning protocol was applied. Three TAR cloning vectors were designed to contain upstream sequences of group A, B and C var genes as 5′ targeting sites as well as the ATS domain as 3′ targeting site. In addition, they contained a yeast centromere, an autonomously replicating sequence (ARS), positive and negative selectable markers as well as an ori and ampR gene for replication in E. coli cells. Each of the three vectors was co-transformed with genomic DNA of the ITG2F6 strain into yeast spheroplasts. Homologous recombination within the spheroplasts resulted in circular yeast plasmids presumably containing a single var gene (Figure 1). We performed three independent experiments for each of the three TAR cloning vectors and obtained a total of 205 TAR clones of which 46 resulted from recombination with pEB_upsA_ATS, 91 from recombination with pEB_upsB_ATS, and 68 from recombination with pEB_upsC_ATS. This proportion is in accordance with the known distribution of var groups within the genomes of 3D7, IT4/25/4 and HB3 (Table S1).
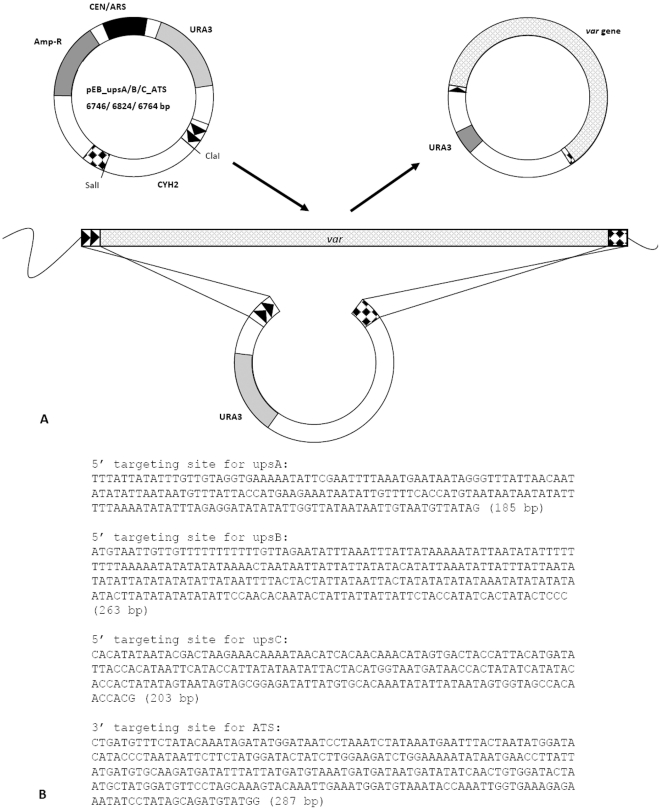
Figure 1. Construction of the var TAR vectors.
(A) TAR cloning vectors pEB_upsA/B or C_ATS were constructed from the general TAR vector pEB2 (M. Becker and E.J. Louis, unpubl.). The 5′ targeting sites upsA (185 bp), upsB (263 bp) or upsC (203 bp) are indicated by black arrows. The 3′ targeting site is identical in all three TAR cloning vectors and contains part of the ATS region (287 bp) indicated with black squares. The yeast centromere and ARS are labelled as CEN and ARS, the positive selectable marker gene URA3 is indicated as light grey box (URA3), the negative selectable marker CYH2 with a white box (CYH2), and the Ampicillin resistance gene with a dark grey box (Amp-R). SalI and ClaI restriction sites are indicated. Homologous recombination between the targeting sites and P. falciparum genomic DNA results in the cloning of a var gene flanked by those sites as a circular molecule. (B) 5′ and 3′ targeting sequences used in the TAR cloning vectors pEB_upsA/B or C_ATS.
PCR screening and Southern blot analysis of TAR clones
In an initial PCR screen, using primers for the most conserved DBL1alpha domain, 48% of TAR clones were identified as positive, i.e. containing a var gene sequence. Subsequent Southern blot analysis conducted on a subset of 104 TAR clones identified 95 putative positive TAR clones (an example is shown in Figure 2), of which only 74 had been positive for the DBL1alpha domain by PCR. This demonstrates that DNA hybridization can reveal more positive clones than PCR due to variation in the primer region. Yeast colonies resulting from transformation with ‘empty’ vector without insert produced a band of approximately 6.0 kb (vector pEB upsA-ATS was used as negative control, Figure 2). We considered all clones larger than this as positive, taking into account the possibility that partial var genes could also be isolated. The additional 21 identified by Southern blotting may also contain var genes that are more divergent. A total of 119 putative TAR clones were identified with either or both methods within the 3 sets of experiments. TAR clone sizes ranged from less than 6 kb to approximately 30 kb (examples shown in Table 2). Sequencing of a 30 kb clone generated two contigs which could be linked by gap closure PCR yielding a total length of 11.5 kb which was further confirmed by RFLP analysis (see below).
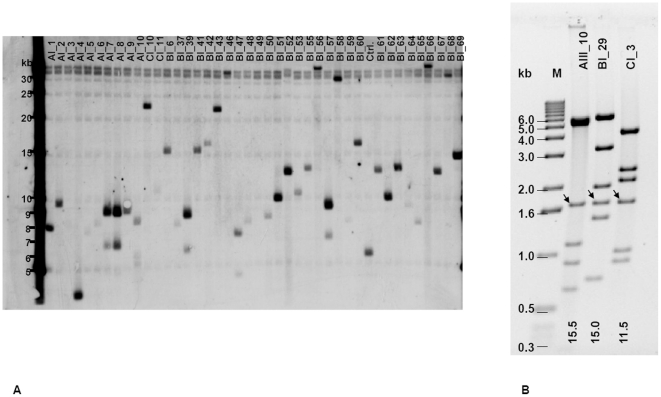
Figure 2. Southern blot analysis of var clones.
(A) Southern blot example of 41 samples of NotI-digested genomic DNA of yeast TAR clones probed with vector-backbone. One lane contains a negative control (ctrl). (B) Restriction fragment pattern of TAR clones upsAIII_10, upsBI_29 and upsCI_3 digested with NotI and NdeI. The vector backbone yielded a band of 1600 bp and is marked with an arrow. The molecular marker is indicated. The upper band of the upsAIII_10 clones represents a double band.
Table 2. Comparison of selected TAR clones by Sanger sequencing of 5′ and 3′ ends of inserts and size analysis by Southern blot (a) and RFLP (b).
| TAR clone | Blast result 5′sequence (gene-ID) | Blast result 3′ sequence (gene-ID) | var group | TAR clone sizea | TAR clone sizeb |
| upsAI_2 | IT4_var3 | IT4_var3 | upsA | 9.5 kbp | 8 kbp |
| upsAI_9 | PFD0020c | VARC2 | upsA | 9.5 kbp | 8.6 kbp |
| upsAIII_10 | TM180var2 | IT4_var54 | upsA | 15 kbp | 15.5 kbp |
| upsBI_1 | IT4_var54 | IT4_var54 | upsB | 13.5 kbp | 12 kbp |
| upsBI_2 | MAL7P1.55 | PFI0005w | upsBsh | 13.5 kbp | 11 kbp |
| upsBI_6 | IT4_var41 | IT4_var26 | upsB | 16 kbp | 16 kbp |
| upsBI_8 | Var13.1 | IT4_var28 | upsB | 17 kbp | 17 kbp |
| upsBI_11 | Dd2_LT141 | IT4_var13 | upsB | 16 kbp | 15 kbp |
| upsBI_12 | IT4_var26 | IT4_var26 | upsB | 13 kbp | 12 kbp |
| upsBI_14 | IT4_var13 | Dd2_LT141 | upsB | 16 kbp | 17 kbp |
| upsBI_17 | IT4_var11 | IT4_var11 | upsB | 14 kbp | 13.5 kbp |
| upsBI_21 | var-3 | var-3 | upsB | 15.5 kbp | 14 kbp |
| upsBI_23 | - | IT4_var24 | ? | 10 kbp | 11.5 kbp |
| upsBI_29 | IT4_var13 | Dd2_LT141 | upsB | 16.5 kbp | 15 kbp |
| upsBI_31 | var (EU787536) | IT4_var24 | upsB | 13.5 kbp | 10.5 kbp |
| upsBI_32 | PfEMP1(PFU31083) | FCR3-varT11-1 | upsB | 15 kbp | 15 kbp |
| upsBI_33 | var (EU787596) | IT4_var24 | upsB | 10 kbp | 11 kbp |
| upsBI_35 | var-3 | PFI0005w | upsB | 13 kbp | 10 kbp |
| upsBI_38 | IT4_var54 | IT4_var54 | upsB | 13 kbp | 15 kbp |
| upsBI_41 | var-3 | var-3 | upsB | 15.5 kbp | 13 kbp |
| upsBI_61 | var (AY462592) | IT4_var36 | upsB | 13 kbp | 13 kbp |
| upsBII_1 | IT4_var33 | IT4_var33 | upsB | n. d. | 12 kbp |
| upsBII_3 | var13.1 | var13.1 | upsB | n. d. | 12.7 kbp |
| upsCI_3 | IT4_var23p | PF07_0049 | upsC | 28 kbp | 12.5 kbp |
| upsCI_10 | Var (EU787639) | PFD0995c | upsC | 23 kbp | 10 kbp |
| upsCI_7 | IT4_var23p | PFL1960w | upsC | 14 kbp | 12 kbp |
| upsCI_13 | IT4_var23p | var13.1 | upsC | >30 kbp | 12.5 kbp |
| upsCII_6 | PF07_0051 | PFL1970w | upsC | n. d. | 12.5 kbp |
| upsCII_14 | PF07_0051 | IT4var24 | upsC | n. d. | 13 kbp |
| upsCII_10 | IT4_var40 | PFD1015c | upsB | n. d. | 13.5 kbp |
| upsCII_25 | IT4_var23p | PF07_0049 | upsC | n. d. | 12.5 kbp |
| upsCIII_12 | - | Varc2 | ? | 13 kbp | 12.5 kbp |
var groups were determined by sequence alignment of targeting sites and from 5′ BLAST analysis. 5′ sequences of clones upsBI_23 and upsCIII_12 could not be determined consequently var group affiliation could not be determined. n.d. = not done.
Restriction fragment Length polymorphism (RFLP) of selected TAR clones
In order to perform RFLP analysis, we transformed E. coli with DNA from yeast containing several candidate TAR clones. Despite the large size of the plasmids and the toxicity of yeast DNA to bacteria, we obtained 36 E. coli clones containing the TAR plasmids. To analyze the variability of the isolated TAR clones, we performed RFLP using restriction enzymes NotI and NdeI. The GC-rich NotI restriction site is unlikely to cut the AT-rich P. falciparum var gene sequence. There is a single NotI and one NdeI restriction site in the vector backbone of pEB2 which results in a distinct characteristic band of 1600 bp in all TAR clones. Screening the published 3D7 and IT4/4/25 var gene sequences, NdeI restriction sites were found to be present at one to eight locations in individual var genes, resulting in expected distinct banding patterns for different var gene inserts. All 36 E. coli clones were analyzed in this way. All upsA clones showed different NotI/NdeI digestion patterns indicating that different upsA var genes have been isolated. For upsB 23 TAR clones were analyzed, resulting in 20 unique patterns. One pattern was represented three times and one pattern was represented twice. The restriction digest of ten upsC clones revealed six patterns of which four were unique, one was represented four times and one twice.
These RFLP data demonstrate a high variability of isolated var genes. Although var gene IT4_var23p (Table 2) was represented four times, no bias towards cloning certain var genes was observed which could lead to the isolation of a restricted set of var genes.
Figure 2 shows the RFLP pattern of TAR clones upsAIII_10, upsBI_29 and upsCI_3, representative of all other examined clones. The total size of the bands summed to 15.5 kbp for upsAIII_10, 15 kbp for upsBI_29 and 11.5 kbp for upsCI_3. For upsAIII_10 and upsBI_29 this is consistent with the results obtained from Southern blot analysis and shotgun sequencing (Table 2 and section below), for clone upsCI_3 it is consistent with shotgun sequencing (section below).
Sequence analysis of TAR clones
We sequenced a number of PCR-products obtained directly from the colony screening o TAR clones using both forward and reverse DBL1alpha -primers. As the degree of DBL1alpha conservation is only about 50% [17], annealing of the primers to different var genes is probably not optimal. The AT-richness of the var genes may explain why low sequence quality was obtained in some instances. However, BLAST analysis of all sequences confirmed the presence of P. falciparum var gene sequences, demonstrating that the TAR clones contained var gene inserts.
To confirm that nearly complete var genes were cloned, a representative clone for each of the different RFLP patterns was selected and 5′ and 3′ sequences were generated using primers located on either recombination site. Correct 5′ and 3′ var gene sequences were obtained for 30 clones. For two clones only a correct 3′ sequence was obtained. Three sequences represented group A, 20 sequences group B and nine group C var genes (Table 2). All sequences extended beyond the targeting sequence confirming successful recombination and cloning. As expected from the RFLP results, BLAST analysis gave identical hits for some TAR clones (Table 2). One clone, isolated with the upsC 5′ targeting sequence showed higher similarity to a group B var gene (PlasmoDB accession number PFD1015c) confirming previously observed limited fidelity within these two groups of var genes [16].
For each var group, we sequenced one yeast clone by deep sequencing (Illumina) and the sequences aligned well with the 5′ and 3′ sequences obtained by Sanger sequencing of the plasmids. For clone upsAIII_10 and clone upsBI_29 we were able to assemble all reads de novo into a single contig. We were able to identify the backbone of the TAR cloning vector pEB2, the 5′ targeting sites upsA and upsB, and the 3′ targeting site ATS in both contigs. BLAST searches were performed with the complete isolated var sequences against the NCBI non-redundant nucleotide database. Clone upsAIII_10 consisted of 11596 bp. BLAST search identified a partially sequenced P. falciparum var gene of the FCR3 strain (Accession number AJ133811) with 99% identity (13 mismatches over 9024 bp).
BLAST search of TAR clone upsBI_29 identified the IT4_var13 var gene (Accession number EF158072) with an identity of 99%. This similarity was expected since the strain ITG2F6 used here is a subclone of strain IT4/4/25. IT4_var13 is a group B var gene confirming the specificity of the upsB targeting sequence.
Sequencing of the upsC TAR clone upsCI_3 revealed two contigs each overlapping at one end with the pEB2 backbone. The gap between the contigs was closed by PCR using primers CI_3_pF and CI_3_pR (Table 1) corresponding to a sequence 90 bp before the unmatched end of either contig. The 500 bp fragment (Figure S1) resulted in an extremely AT rich sequence. The 5′ end generated with the forward primer showed a 100% match with the last 49 bp of contig 1 and similarly, the sequence generated with the reverse primer matched to the first 45 bp of the of contig 2 (Supplementary Figure 1). Further analysis revealed that the missing sequence stretch lies within the intron sequence. BLAST searches of the gap sequence resulted in a hit on the intron of var gene PFI0005w (Accession number AL844508). The complete sequence of clone upsCI_3 showed highest similarities with the partial IT4_var23 var gene (Accession number EF158077) belonging to group C var genes. This var gene has only been sequenced partially over 2049 bp displaying a 99% identity to the upsCI_3 TAR clone. The 3′ end of clone upsCI_3 showed an 85% identity with another partially sequence var gene (VARC1, Accession number AF100791).
Table 1. Primers used for targeting, screening, sequencing, and gap closure.
| primer name | sequence 5′to 3′ |
| upsA_fw | tataatggatcctttattatatttgttgtaggtgaaa |
| upsA_rev | taatataagcttctataacattacaattattataacc |
| upsB_fw | tataatggatccatgtaattgttgtttttttttttgttag |
| upsB_rev | taatataagcttgggagtatagtgatatggtag |
| upsC_fw | tataatggatcccacatataatacgactaag |
| upsC_rev | taatataagcttcgtggttgtggctac |
| ATS_fw | tattatgtcgacctgatgtttctatacaaatag |
| ATS_rev | ataaatgtcgacgaattcccatacatctgctatagg |
| DBL1alpha _fw | gcaccgaagttttgcagatatwgg |
| DBL1alpha _rev | aartcttckgcccattcctcgaacca |
| CI_3_pF | ggatatatatatggatttacatg |
| CI_3_pR | gggtatatatatatatgtatgtg |
Restriction sites are underlined. W denotes nucleotides A or T, R denotes A or G, and K denotes G or T.
All three isolated clones contained an open reading frame beginning with a methionine and were interrupted by an intron. The 3′ targeting sites of all three TAR cloning vectors do not cover the complete ATS domain, therefore the isolated var genes lack a stop codon in the second exon.
Discussion
We present the successful adaptation of the transformation-associated recombination (TAR) method [22] to isolate sets of individual var genes from the P. falciparum ITG2F6 strain. We obtained a library of 205 independent TAR clones, 98 (48%) of which tested positive for the presence of var gene sequence using the most conserved DBL1alpha domain in colony PCR screening. The low percentage of positives is a reflection of the limited conservation (around 50%) of the diagnostic DBL1alpha domain. PCR screening is adequate for first and fast results but is not reliable for the detection of the entire repertoire in the library. Using Southern blot analysis on a subset of 104 yeast clones 95 putative positives (91%) were detected, indicating that the library is likely to contain almost exclusively total or partial var genes. To further analyze these clones, 36 TAR clones were selected, transformed into E. coli cells and plasmids analyzed by restriction fragment length polymorphism (RFLP) and sequencing. As proof of principle, we further showed that pooling and bar-coding of three clones containing P. falciparum var genes allowed the subsequent de novo reassembly after deep sequencing (Illumina).
The use of yeast artificial chromosomes to clone large fragments of DNA dates back to the late 80s when Silverman and colleagues cloned segments of human chromosomes and specifically members of gene families [22], [31], [32]. Later, this technique was successfully applied to specifically clone human centromeric regions [23], [24]. TAR cloning was also used for gap closure in difficult areas in the human genome project [25]. With the development of higher sequencing capacity, it became feasible to target specific gene families by TAR cloning and an excellent example was the specific cloning of T. brucei brucei, T.b. gambiense and T. equiperdum vsg expression sites [26], [27].
The majority of var genes of Plasmodium falciparum are located in subtelomeric locations which represent approximately 10% of the whole genome. These areas are highly diverse and thought to undergo frequent recombination [33]. In addition, var genes are composed of repetitive domains with a high AT content. Sequencing projects struggle with the assembly of telomeric ends due to the limited information content in small sequence fragments from these areas. This poses large problems in the correct assembly of large multi-gene family genes such as var genes. Here we have shown that TAR cloning, which would generate clones containing only a single full-length var gene would greatly facilitate sequencing of the var gene repertoire of individual isolates overcoming the many ambiguities associated with such repetitive stretches.
To prove the feasibility and the principle of this approach we aimed at the specific isolation of var genes from the ITG2F6 parasite strain. We searched for conserved regions in the 5′ upstream regions which are used to define three groups and which show a relatively high similarity within but not between the groups [12], [13]. Based on the identified sequences we constructed for each of the groups a TAR cloning vector. We obtained 46 clones of group A var genes, 91 of group B var genes, and 68 clones of group C var genes and this distribution is broadly similar to the observed distribution of var genes in P. falciparum strains 3d7, It4, and HB3 [13], [30] (Supplemental Table 1). Sequence analysis of a number of obtained TAR clones demonstrated that most of the isolated var genes derived from the group which the cloning vectors had targeted, indicating that the selected targeting sequences occurred sufficiently specific to isolate var genes of a defined group. The one exception observed (one B clone obtained with the upsC vector) might be due to some alignment between sequences used as target sites for upsB and upsC var genes, which exhibit 44% identity.
Reliable screening of positive clones by PCR from colonies directly proved to be difficult. PCR amplification of a 400 bp fragment within the DBL1alpha domain, which is present in all known var genes (except in var2), gave a significant number of false negative clones (around 20%) based on subsequent Southern blot analysis. Although PCR tolerates some diversity, sequence conservation between DBL domains of as little as 52% [17] might be responsible for the missed sequences, particularly from colonies directly. In contrast, Southern blotting of total genomic or plasmid DNA identified many more potential positive clones. We considered all TAR clones with a size >6.5 kb as positive and demonstrated that all clones with total sizes above 9 kb contained var genes as confirmed by sequencing of the 5′ and 3′ ends of the insert.
RFLP analysis and sequencing of the 5′ and 3′ termini indicated that we isolated a broad range of different var genes. However, BLAST analysis using the sequence fragments and RFLP pattern also suggested some clones to be identical. The 5′ sequences of four TAR clones, i.e. upsCII_25, upsCI_3, upsCI_7 and upsCI_13 resulted in the same BLAST hit (Table 2) and three of those, upsCII_25, upsCI_3, upsCI_13, also revealed a similar RFLP pattern. The fourth clone, upsCI_7, had a different digestion pattern and thus was different in its overall sequence indicating that a large and diverse number of complete var genes were cloned.
As proof principle, and to show that de novo assembly of var genes is possible with massively parallel sequencing, we analyzed three bar-coded TAR clones by Illumina sequencing. It is well known that AT richness and repeat structure of var genes poses large difficulties in the assembly of short sequence reads. However, we were able to assemble two out of three clones completely de novo. Clone upsBI_29 resulted in a single contig with 99% sequence identity to the coding sequence of the IT4var13 gene suggesting that the de novo assembled contig was correct. This gene previously has been isolated from the IT4/25/5 strain which is the parental strain of ITG2F6. Similarly, clone upsAIII_10 showed sequence identity of 99% to a var gene of the FCR3 strain associated with CSA binding. BLAST analysis found homologies in a stretch of approximately 1 kb in the 3′ sequence of a homologous incompletely sequenced var gene of the IT4/25/5 strain. Correct sequence assembly is confirmed by high sequence similarities to partially sequenced var genes derived from closely related strains. Using TAR cloning we were able to determine the full length sequence of these var genes. It is noteworthy that intron sequences in both TAR clones differed in length from the intron sequence of the annotated genes. This is likely to be due to the extremely high AT richness of the introns. The third clone, upsCI_3, comprised two contigs which we were able to link by PCR and Sanger sequencing. The inconsistency between the size of this clone determined by Southern analysis and the RFLP and sequence analysis could be due to a variety of issues such as a plasmid dimer in yeast. The PCR product spanning the gap was corresponded to the intron and was as such extremely AT-rich. Therefore, we could not fully determine the length of the gap sequence, highlighting the difficulties to assemble such DNA domains in the genome sequencing projects.
In conclusion, we have shown that TAR cloning can be used to specifically clone members of gene families in P. falciparum and this technology can now be applied to field samples to study the full diversity of var genes. It might be possible to clone full length var genes from cDNA which would allow association of expressed var genes with distinct phenotypes. This could provide enormous insight into the presence of phenotype-associated var genes. Having the predicted amino acid sequence of such genes could shed light onto the true associations with disease, as full length PfEMP1 molecules might confer different binding affinities than cloned short fragments out of the protein context. TAR cloning could become a major tool for functional elucidation of multigene families in P. falciparum. It also could be applied to specifically isolate members of other multigene-families within the P. falciparum genome, such as the repetitive interspersed family (rif) or the subtelomeric open reading frame (stevor) genes [34]-[36]. Similar to var genes, these genes often cluster at subtelomeric regions. TAR cloning could be applied to isolate telomere ends and larger chromosomal regions and thus increase our knowledge on these important regions in P. falciparum.
Methods
P. falciparum strain
Parasites of P. falciparum strain ITG2F6 were cultured at 5% hematocrit as previously described [37], using RPMI medium supplemented with 0.5% Albumax [38]. Cells were lysed in 0.03% saponin in PBS for 10 minutes on ice, centrifuged at 4000 x g for 10 minutes and washed in PBS until the supernatant was clear. The parasite containing pellet was subjected to proteinase K digestion and genomic DNA was subsequently prepared by phenol/chloroform precipitation [39].
S. cerevisiae strain
The S. cerevisiae strain TYC1 (genotype: MATa, ura3-52, leu2Δ1, cyh2r, containing a knockout of DNA Ligase 4) was used as the yeast host in TAR experiments as previously published [26].
Construction of var TAR cloning vectors
var TAR cloning vectors pEB_upsA-ATS, pEB_upsB-ATS and pEB_upsC-ATS, respectively were constructed from the basic TAR cloning vector pEB2 (M M Becker and E J Louis, unpublished). They contain the yeast selectable marker URA3, the negative-selection marker CYH2 [40], a yeast centromere and an autonomously replicating sequence (ARS). In addition the vector contains an origin of replication and the ampR gene for growth and selection in E. coli (Figure 1)
For the use as 5′ targeting sites, homologous regions of the 5′ untranslated regions of group A, B, and C var genes were individually cloned into vector pEB2 upstream of the CYH2 gene. A 292 bp fragment homologous to the acidic terminal sequence (ATS) of var genes was cloned into all three vectors downstream of the CYH2 gene as 3′ targeting site (Figure 1). The 5′ homologous regions were 185 bp long for the group A genes, and 263 and 203 bp for the group B and C var genes, respectively. All 5′ targeting sites were chosen based on alignments of partially sequenced field samples (Accession numbers EU787517.1 – EU787984.1, AY462581.1 – AY462851.1) and the published 3d7 var gene sequences. For the 3′ targeting site only 3d7 var gene sequences were aligned, as no sequence information of field samples was available. The most conserved sequence stretches were chosen as 5′ and 3′ targeting sites, and were amplified by PCR from ITG2F6 genomic DNA using the primers shown in Table 1. Initially, all amplified targeting sequences were cloned into a pGEXT-vector and several clones per group were sequenced. For each target site, the sequence that occurred with the highest frequency and highest similarity to the original consensus sequence, was moved into pEB2, to form vector constructs as shown in Figure 1. The 5′targeting sites for group A, group B, and group C var genes were cloned using restriction sites BamHI and HindIII into pEB2. The 3′targeting site containing a sequence stretch homologous to the ATS domain was cloned via SalI/XhoI into pEB2. Clones were sequenced again to confirm the correct orientation of the targeting sites. Prior to transformation into yeast spheroplasts all vectors were digested with ClaI and SalI restriction enzymes to linearize the plasmids and to release the CYH2 gene.
TAR procedure
The TAR cloning procedure was essentially performed according to Kouprina and Larionov [29] with the following modifications: Yeast cells were incubated with zymolyase solution (10 µg/ml) for 30 min at 30°C, for each transformation reaction 450 µl of yeast spheroplast solution was used, 4.5ml PEG 8000 solution was used and spheroplasts were resuspended in 1ml SOS solution. Spheroplasts from each transformation reaction were mixed with 15 ml melted synthetic complete agarose medium lacking uracil but containing 1 M Sorbitol and 10 µg/ml cycloheximide and poured onto plates containing the same medium. This allows for positive selection of the TAR vector that provides URA3 function. The addition of cycloheximide prevents transformation of empty plasmids, which remain circular due to incomplete ClaI and SalI restriction digest, as those vectors still maintain the CYH2 gene that confers dominant sensitivity to cycloheximide. A full manual can be downloaded from www.nottingham.ac.uk/biology/people/louis/principleoftarcloning.
Identification of var gene positive TAR transformants
TAR transformants, grown selective medium, were initially screened by colony PCR using primers for the DBL1alpha domain (Table 1). Single yeast colonies were suspended in a mix of 10 µM pF_DBL1alpha, 10 µM pR_DBL1alpha (Table 1), 0.4 µl Taq and 0.9 µl 11.1xPCR-buffer (45 mM Tris-HCl (pH 8.8), 11 mM ammoniumsulphate, 4.5 mM MgCl2, 6.7 mM 2-mercaptoethanol, 4.4 mM EDTA (pH 8.0), 113 µg/ml BSA, 1 mM dATP, dTTP, dCTP and dGTP) and adjusted with H2O to a final volume of 10 µl. PCR conditions were as follows: 5 min at 95°C, 40 cycles of 30 sec 94°C, 40 sec 57°C and 2 min 72°C and a final step of 5 min at 75°C.
All yeast TAR clones were propagated on selective medium and yeast genomic DNA was isolated by phenol/chloroform purification (as described in [26]) with subsequent precipitation. Alternatively, yeast plasmid DNA was directly purified using the Zymoprep II™ Yeast Plasmid Miniprep Kit (Zymo Research) or the QIAprep Spin Miniprep Kit (Qiagen).
Southern Blot analysis
Genomic DNA of yeast TAR clones was digested with NotI and separated on 1% agarose gels in 0.5 x TBE using a CHEF mapper system (BIORAD) with the following conditions: forward: 9 V/cm, 10 s initial switch, 40 s final switch, reverse: 6 V/cm, 10 s initial switch, 40 s final switch, for 24 hrs at 14°C.
Southern blot, hybridization and detection were performed as per manufacturer's instruction for non-radioactive nucleic labeling and detection (Roche Diagnostics) using Blocking reagent (Roche), CDP-Star (PerkinElmer or Roche), Maleic Acid (Sigma). The probe consisted of fluorescein-labeled vector backbone (pGEM7, Promega) using Fluorescein-High Prime labeling kit (Roche) and was immunologically detected using Anti-fluorescein-AP, Fab fragments (Roche) on positively charged nylon membranes (Roche).
Transformation of positive yeast clones into E. coli and Restriction Fragment Length Polymorphism (RFLP) analysis
Genomic DNA of individual yeast clones was prepared as described above and transformed into E. coli PMC103 either by electroporation or by chemical transformation into ultra competent DH5α cells. Plasmid DNA from bacterial cells was purified using the QIAprep Spin Miniprep Kit (Qiagen).
For RFLP analyses, purified plasmid DNA, of individual clones, were digested with NotI and NdeI. The digests were separated on a 0.7% agarose gel and visualized by ethidiumbromide staining. The vector backbone contains one restriction site each for NotI and NdeI and the number of NdeI-restriction sites varies for different var genes, thereby creating a distinct band for the vector backbone and a unique pattern of bands for individual var genes. To determine the insert quality, we sequenced the 5′ and 3′ ends of each isolated TAR clone using plasmid DNA (Table 2). In addition, the PCR products obtained from the DBL1alpha colony screen of yeast clones were sequenced. All sequencing was conducted at Macrogen Inc., Korea.
Next-generation sequencing of three TAR clones
TAR clones upsAIII_10, upsBI_29 and upsCI_3 were selected for tagged deep sequencing. After transformation in E. coli, high plasmid concentrations were prepared using the PowerPrep™ HP Plasmid Maxiprep (Marligen Biosciences). Initial sequence analyses were performed by Fasteris SA, Switzerland. Briefly, individual clones were sheared by Fragmentase treatment (NEBNext™ dsDNA Fragmentase™, New England Biolabs) according to the manufacturer's instructions, purified via Qiagen columns and fragments of each clone were labeled with distinct bar-coded adapters to distinguish the reads of the three different TAR clones. High-throughput sequencing was performed on Genome Analyzer GAII, in 1 PE channel; obtaining 2 x 38 bp reads. Bioinformatic analysis included de novo assembly, assembly overlap and validation using the following software: VELVET, version 0.7.54, SeqMan, version 8 and MAQ, version 0.7.1.
The obtained sequences of clones upsAIII_10, upsBI_29 and upsCI_3 have been deposited with NCBI with the following Accession numbers HM358637-39.
Sequence analysis
All alignments were performed with the ClustalW multiple alignment tool (www.ebi.ac.uk/Tools/clustalw2) at default parameters. BLAST analyses were performed against the NCBI nucleotide database or against the non-redundant protein sequences database at NCBI. A The open reading frame of three complete sequences were analyzed using the translation tool of the ExPASy proteomics server (www.expasy.ch) from the Swiss Institute of Bioinformatics (SIB) [41] and the InterProScan Sequence Search of EBML-EBI.
Supporting Information
Gap closure of clone upsCI_3. (A) Diagram of the structure of the clone with gap and PCR primers designed shown. (B) PCR product covering the gap. (C) Sequence analysis of the PCR product showing AT richness.
(TIF)
Distribution of var genes within the three var groups upsA, upsB and upsC obtained by the TAR experiment compared with P. falciparum strains IT4/25/4, 3d7 and HB3 [19], [30]. * For the 3d7 strain, the number of upsB var genes include B/A and B/C var groups [19].
(DOCX)
Acknowledgments
We thank members of the HPB and EJL laboratories for comments and discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Swiss National Science Foundation [grant number 3100A0-118456] for AG and HPB, and by the Wellcome Trust for MMB and EJL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. The world health report 2008: primary health care now and more than ever. 2008. Available at: http://www.who.int/whr/2008.
- 2.Baruch DI, Gormely JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci U.S.A. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen ATR, Magistrado P, Sharp S, Joergensen L, Lavstsen T, et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J Exp Med. 2004;199:1179–1190. doi: 10.1084/jem.20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chookajorn T, Ponsuwanna P, Cui L. Mutually exclusive var gene expression in the malaria parasite: multiple layers of regulation. Trends Parasitol. 2008;24:455–461. doi: 10.1016/j.pt.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, et al. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bull PC, Lowe BS, Kortok M, Marsh K. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. 1999;67:733–739. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyes SA, Kraemer SM, Smith JD. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryotic Cell. 2007;6:1511–1520. doi: 10.1128/EC.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–380. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Voss TS, Kaestli M, Vogel D, Bopp S, Beck H. Identification of nuclear proteins that interact differentially with Plasmodium falciparum var gene promoters. Mol Microbiol. 2003;48:1593–1607. doi: 10.1046/j.1365-2958.2003.03528.x. [DOI] [PubMed] [Google Scholar]
- 11.Voss TS, Healer J, Marty AJ, Duffy MF, Thompson JK, et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 12.Gardner MJ, Hall N, Fung E, White O, Berriman M, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavstsen T, Salanti A, Jensen ATR, Arnot DE, Theander TG. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar J. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry AE, Leliwa-Sytek A, Tavul L, Imrie H, Migot-Nabias F, et al. Population genomics of the immune evasion (var) genes of Plasmodium falciparum. PLoS Pathog. 2007;3:e34. doi: 10.1371/journal.ppat.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaestli M, Cockburn IA, Cortés A, Baea K, Rowe JA, et al. Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J Infect Dis. 2006;193:1567–1574. doi: 10.1086/503776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk N, Kaestli M, Qi W, Ott M, Baea K, et al. Analysis of Plasmodium falciparum var genes expressed in children from Papua New Guinea. J Infect Dis. 2009;200:347–356. doi: 10.1086/600071. [DOI] [PubMed] [Google Scholar]
- 17.Rowe JA, Kyes SA. The role of Plasmodium falciparum var genes in malaria in pregnancy. Mol Microbiol. 2004;53:1011–1019. doi: 10.1111/j.1365-2958.2004.04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe JA, Kyes SA, Rogerson SJ, Babiker HA, Raza A. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J Infect Dis. 2002;185:1207–1211. doi: 10.1086/339684. [DOI] [PubMed] [Google Scholar]
- 19.Lavstsen T, Magistrado P, Hermsen CC, Salanti A, Jensen ATR, et al. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malar J. 2005;4:21. doi: 10.1186/1475-2875-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyriacou HM, Stone GN, Challis RJ, Raza A, Lyke KE, et al. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol Biochem Parasitol. 2006;150:211–218. doi: 10.1016/j.molbiopara.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rottmann M, Lavstsen T, Mugasa JP, Kaestli M, Jensen ATR, et al. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun. 2006;74:3904–3911. doi: 10.1128/IAI.02073-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larionov V, Kouprina N, Graves J, Chen XN, Korenberg JR, et al. Specific cloning of human DNA as yeast artificial chromosomes by transformation-associated recombination. Proc Natl Acad Sci U.S.A. 1996;93:491–496. doi: 10.1073/pnas.93.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancilla MR, Tainton KM, Barry AE, Larionov V, Kouprina N, et al. Direct cloning of human 10q25 neocentromere DNA using transformation-associated recombination (TAR) in yeast. Genomics. 1998;47:399–404. doi: 10.1006/geno.1997.5129. [DOI] [PubMed] [Google Scholar]
- 24.Kouprina N, Ebersole T, Koriabine M, Pak E, Rogozin IB, et al. Cloning of human centromeres by transformation-associated recombination in yeast and generation of functional human artificial chromosomes. Nucleic Acids Res. 2003;31:922–934. doi: 10.1093/nar/gkg182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouprina N, Leem S, Solomon G, Ly A, Koriabine M, et al. Segments missing from the draft human genome sequence can be isolated by transformation-associated recombination cloning in yeast. EMBO Rep. 2003;4:257–262. doi: 10.1038/sj.embor.embor766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker M, Aitcheson N, Byles E, Wickstead B, Louis E, et al. Isolation of the repertoire of VSG expression site containing telomeres of Trypanosoma brucei 427 using transformation-associated recombination in yeast. Genome Res. 2004;14:2319–2329. doi: 10.1101/gr.2955304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young R, Taylor JE, Kurioka A, Becker M, Louis EJ, et al. Isolation and analysis of the genetic diversity of repertoires of VSG expression site containing telomeres from Trypanosoma brucei gambiense, T. b. brucei and T. equiperdum. BMC Genomics. 2008;9:385. doi: 10.1186/1471-2164-9-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kouprina N, Larionov V. TAR cloning: insights into gene function, long-range haplotypes and genome structure and evolution. Nat Rev Genet. 2006;7:805–812. doi: 10.1038/nrg1943. [DOI] [PubMed] [Google Scholar]
- 29.Kouprina N, Larionov V. Selective isolation of genomic loci from complex genomes by transformation-associated recombination cloning in the yeast Saccharomyces cerevisiae. Nat Protoc. 2008;3:371–377. doi: 10.1038/nprot.2008.5. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer SM, Kyes SA, Aggarwal G, Springer AL, Nelson SO, et al. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: comparisons of geographically diverse isolates. BMC Genomics. 2007;8:45. doi: 10.1186/1471-2164-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman GA, Ye RD, Pollock KM, Sadler JE, Korsmeyer SJ. Use of yeast artificial chromosome clones for mapping and walking within human chromosome segment 18q21.3. Proc Natl Acad Sci U.S.A. 1989;86:7485–7489. doi: 10.1073/pnas.86.19.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouprina N, Graves J, Cancilla MR, Resnick MA, Larionov V. Specific isolation of human rDNA genes by TAR cloning. Gene. 1997;197:269–276. doi: 10.1016/s0378-1119(97)00271-0. [DOI] [PubMed] [Google Scholar]
- 33.Freitas-Junior LH, Bottius E, Pirrit LA, Deitsch KW, Scheidig C, et al. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature. 2000;407:1018–1022. doi: 10.1038/35039531. [DOI] [PubMed] [Google Scholar]
- 34.Petter M, Haeggström M, Khattab A, Fernandez V, Klinkert M, et al. Variant proteins of the Plasmodium falciparum RIFIN family show distinct subcellular localization and developmental expression patterns. Mol Biochem Parasitol. 2007;156:51–61. doi: 10.1016/j.molbiopara.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Blythe JE, Niang M, Marsh K, Holder AA, Langhorne J, et al. Characterization of the repertoire diversity of the Plasmodium falciparum stevor multigene family in laboratory and field isolates. Malar J. 2009;8:140. doi: 10.1186/1475-2875-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Q, Cloonan N, Fischer K, Thompson J, Waine G, et al. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol Biochem Parasitol. 1998;97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- 37.Trager W, Jenson JB. Cultivation of malarial parasites. Nature. 1978;273:621–622. doi: 10.1038/273621a0. [DOI] [PubMed] [Google Scholar]
- 38.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature. 1995;374:269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 39.Beck H. Extraction and purification of Plasmodium parasite DNA. Methods Mol Med. 2002;72:159–163. doi: 10.1385/1-59259-271-6:159. [DOI] [PubMed] [Google Scholar]
- 40.Raymond CK, Sims EH, Olson MV. Linker-mediated recombinational subcloning of large DNA fragments using yeast. Genome Res. 2002;12:190–197. doi: 10.1101/gr.205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, et al. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gap closure of clone upsCI_3. (A) Diagram of the structure of the clone with gap and PCR primers designed shown. (B) PCR product covering the gap. (C) Sequence analysis of the PCR product showing AT richness.
(TIF)
Distribution of var genes within the three var groups upsA, upsB and upsC obtained by the TAR experiment compared with P. falciparum strains IT4/25/4, 3d7 and HB3 [19], [30]. * For the 3d7 strain, the number of upsB var genes include B/A and B/C var groups [19].
(DOCX)