Fig. 9.
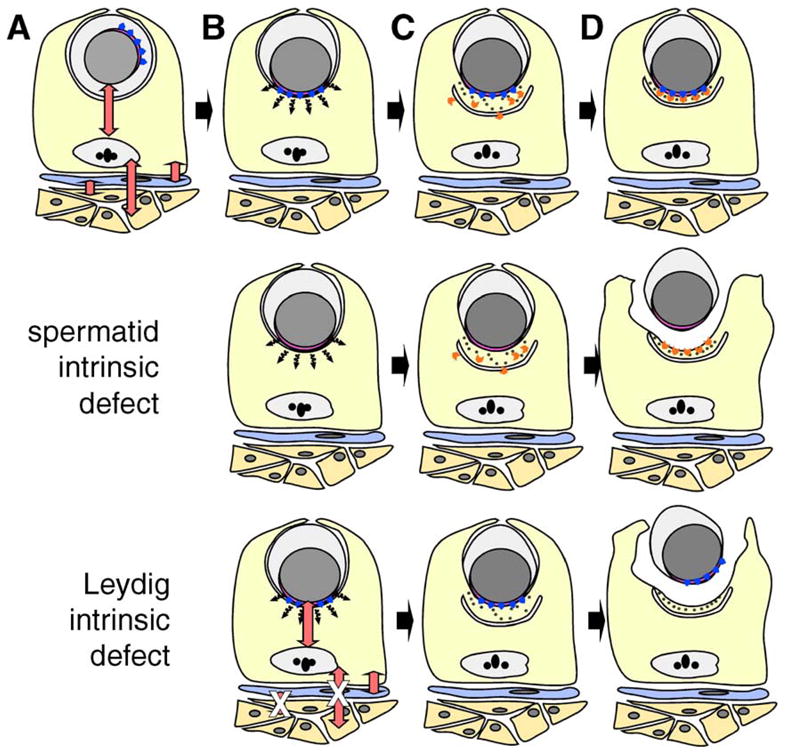
Models to explain the underlying defect in spermatid–Sertoli adhesion in FNDC3A-deficient mice. (A–D) Formation of the ES adhesion junction in wild-type mice. Depicted are Leydig cells (orange), peritubular myoid cell (blue), Sertoli cell (yellow), and a round spermatid. For clarity, other types of germ cells and interstitial cells have been omitted. The red double-headed arrows represent communication between somatic and germ cells within the testis (Mruk and Cheng, 2004b; Saunders, 2003; Skinner, 1991). (A) A round spermatid at step 7 of spermiogenesis. The nucleus (grey) and its tightly apposed acrosome (pink) are located centrally within the round spermatid. The Golgi apparatus is usually located in proximity to the acrosome, which is randomly orientation relative to the basal compartment of the seminiferous epithelium. The blue arrowheads represent proteins localized to the cytosolic face of the acrosome, which are directly or indirectly required within spermatids to form a functional ES with the Sertoli cell. (B) Round spermatid at step 8. The spermatid nucleus with overlying acrosome has moved to abut the plasma membrane. A hypothetical short-range signal (black wavy arrows) transduced from the vicinity of the acrosome instructs the Sertoli cell to (C) assemble components of the ES on the Sertoli side of the ES. Components of the ES within the Sertoli cells are simplified and show the cisternum of ER (the U-shaped structure), hexagonal bundles of filamentous actin (black dots), and other components of the ES required for formation of the adhesive junction (orange chevrons). (D) Completed assembly of the ES between the step 8 spermatid and Sertoli cell. The cartoon depicts the fact that the lateral boundaries of the ES in the Sertoli cell are defined by the lateral boundary of the acrosome in the spermatid. “Spermatid intrinsic defect,” the second row of the figure depicts a model where defective Sertoli–spermatid adhesion results from loss of FNDC3A function within spermatids. The hypothetical signal is transduced from spermatid to Sertoli cell and components of the ES assemble within the Sertoli cell in a location overlying the acrosome. However, as a consequence of loss of FNDC3Awithin spermatids, the germ cell is unable to adhere to the Sertoli cell. In this scenario, FNDC3A could be a structural component required at the acrosomal membrane for function of cell-adhesion molecules associated with the overlying plasma membrane of the spermatid. “Leydig intrinsic defect,” the bottom row depicts a model where defective Sertoli-spermatid adhesion results from loss of FNDC3A function within Leydig cells. In this case, loss of FNDC3A (depicted by white “X” over red double-headed arrows) perturbs Leydig cell function required to generate a functional ES within the Sertoli cell. Abnormal Leydig cell function could affect Sertoli cells directly, or indirectly e.g. via lymphatic endothelial or peritubular myoid cells.
