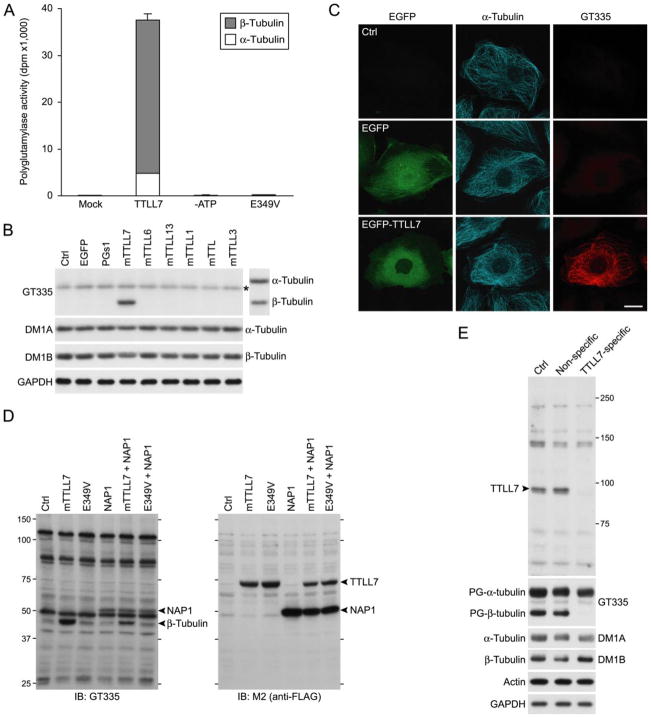
FIGURE 2. TTLL7 is a β-tubulin polyglutamylase.
A, in vitro enzyme activity of recombinant mouse TTLL7. Recombinant mTTLL7 incorporated [3H]glutamate into microtubules with a high preference to β-tubulin in vitro. These activities were lost when ATP was omitted from reaction mixture (–ATP) or when a point mutation causing failure of the enzyme to use ATP was inserted in mTTLL7 (E349V). Enzyme activities are expressed as radioactivity incorporated into microtubules (disintegration per minute; dpm). The result is representative of three independent experiments. B, in vivo enzyme activity of a subset of mouse TTLL proteins. Only mTTLL7 displayed tubulin polyglutamylase activity in HEK293T cells. Polyglutamylated tubulins were detected with a mAb, GT335. The blot was reprobed with DM1A and Tub2.1 to verify migrating positions of both tubulins (displayed on the right of GT335-labeled blot image). Note that the band marked with an asterisk, detected with GT335 in all samples, does not co-migrate with α-tubulin. Amounts of total tubulins were confirmed by reprobing with DM1A and DM1B. Amount of glyceraldehyde-3-phosphate dehydrogenase was monitored as a loading control (Ctrl). The result is representative of three independent experiments. Expression efficacies were shown in supplemental Fig. S2. C, immunocytochemical analysis of effect of overexpressed mTTLL7 in PtK2 cells. Overexpression of EGFP-mTTLL7 in PtK2 cells resulted in polyglutamylation of microtubules. Scale bar, 20 μm. D, in vivo enzyme activity of mTTLL7 on NAP1. To visualize signals other than tubulin labeled by GT335, x-ray film was overexposed. To efficiently detect polyglutamylated NAP1 signal, we overexpressed FLAG-NAP1 with or without mTTLL7. mTTLL7 did not enhance signal intensities of bands other than β-tubulin. Ectopic NAP1 also was not polyglutamylated by mTTLL7. E, knockdown of endogenous TTLL7 and loss of β-tubulin polyglutamylation in primary cultures of SCG neurons. Antiserum raised against TTLL7 detected a protein of ~90 kDa (arrowhead) that disappeared following treatment with siRNA targeted to mTTLL7. Polyglutamylated (PG-) tubulins were detected with mAb GT335. Total α-tubulin and β-tubulin were detected with mAb DM1A and DM1B, respectively.