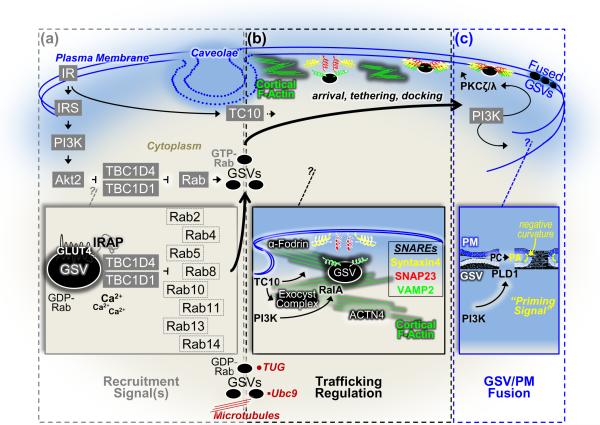
Figure 1.
Schematic illustration of putative signals, cytoskeletal mechanisms, and plasma membrane parameters involved in insulin-stimulated GLUT4-storage vesicle (GSV) exocytosis. (a) Activation of GSVs by insulin requires a PI3K signal involving the upstream IR and IRS activators and the downstream Akt2 target enzyme. TBC1D4 and TBC1D1, substrates of Akt2, have been suggested to couple the PI3K/Akt2 signal to GSVs via its action on one or more critical Rab proteins. The basal intracellular pool of GDP-Rab GSVs shown associated with several putative anchoring systems (e.g., microtubules, Ubc9, TUG) are activated by the suppression of the Rab-GAP activity of TBC1D4/TBC1D1 by Akt2. Several putative Rab proteins, the existence of possible calcium regulation, and mechanisms associating TBC1D4 (and presumably TBC1D1) to the GSV via IRAP have been suggested (see inset). (b) Cortical F-actin, likely originating at the neck region of caveolae PM microdomains, plays a critical role in GSV trafficking. Reorganization of the cortical F-actin meshwork by insulin signaling to TC10 allows GSV/PM arrival, tethering, and docking. A large number of proposed insulin-regulated processes occur in this PM vicinity such as TC10-regulated formation of the exocyst complex and cortical F-actin remodeling, PI3K/RalA-stimulated transition of trafficking GSVs to tethered GSVs, a role of ACTN4 and/or the exocyst complex in tethering, and an α-fodrin-mediated rearrangement of cortical actin filaments in the area of syntaxin 4 to facilitate GSV/PM SNARE protein interaction and docking (see inset). (c) Insulin signaling, through two putative PI3K signals that activate PKCζ/λ and PLD1, prepares GSVs for fusion with the PM. The first PKCζ/λ signal has been implicated in promoting the dissociation of Munc18c from syntaxin4, contributing to the fusion-competent SNARE complex. The second PLD1 signal primes the GSV and PM for fusion by generating PA, which has been suggested to act as a fusogenic lipid in biophysical modeling studies by lowering the activation energy for membrane bending (i.e., negative membrane curvature) during generation and expansion of fusion pores (see inset).