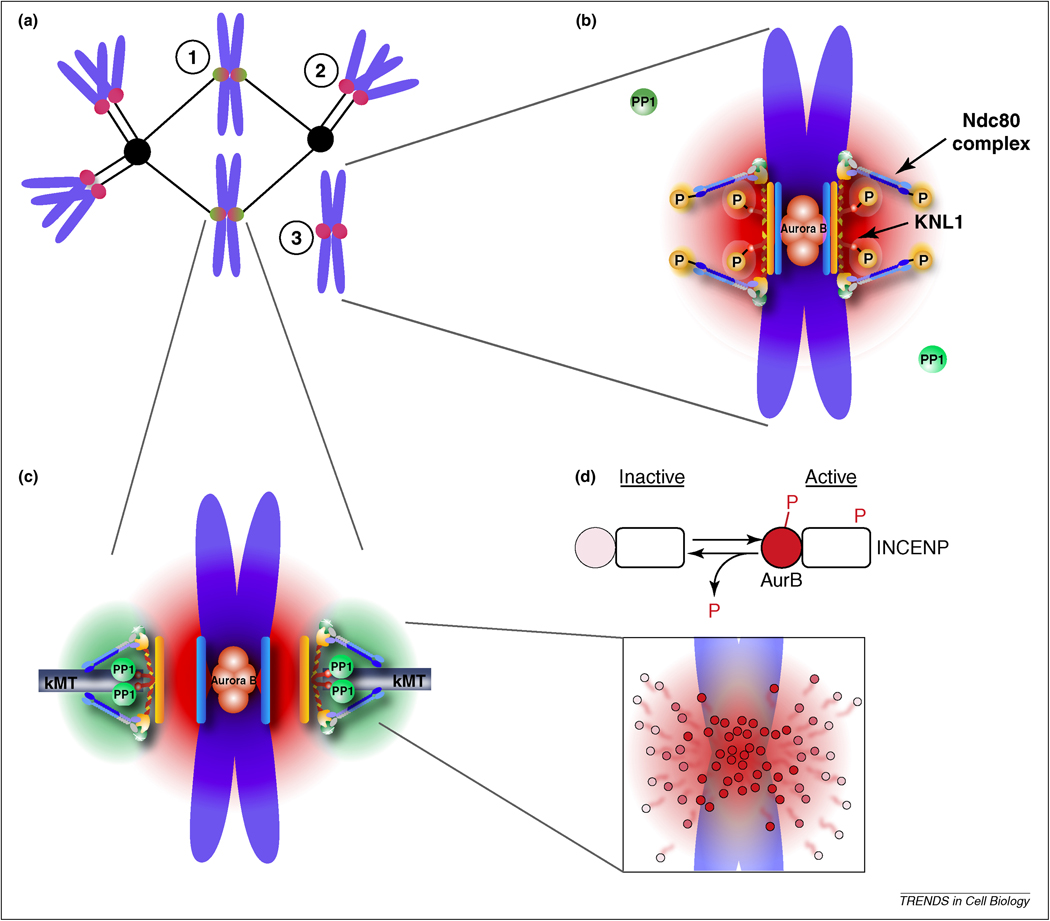
Figure 1. Model for tension sensing by spatial separation of Aurora B from kinetochore substrates.
(a) Cartoon depiction of a spindle with correctly bi-oriented chromosomes (1) and incorrect kinetochore-microtubule attachments including a syntelically attached chromosome (2) or an unattached chromosome (3). (b,c) A phosphorylation gradient is generated by concentration of Aurora B at the inner centromere. Aurora B sites within the KMN network are phosphorylated due to their position within this gradient (red) at incorrect attachments, where tension is low, which destabilizes kinetochore microtubules (b). These substrates are dephosphorylated at correct attachments, where tension is high, because they are positioned farther from the kinase (c). Recruitment of PP1 to the outer kinetochore provides a counteracting gradient of dephosphorylation (green). (d) Model for how a phosphorylation gradient might be generated. Aurora B is activated (dark red circles) at the inner centromere by autophosphorylation, both of Aurora B and INCENP, followed by release and inactivation (lighter circles) by dephosphorylation as Aurora B diffuses away from the inner centromere.