Figure 2. Na+ dependence of the kobs of the slow phase of Na+ binding to thrombin (open circles), factor Xa (black circles) and activated protein C (grey circles).
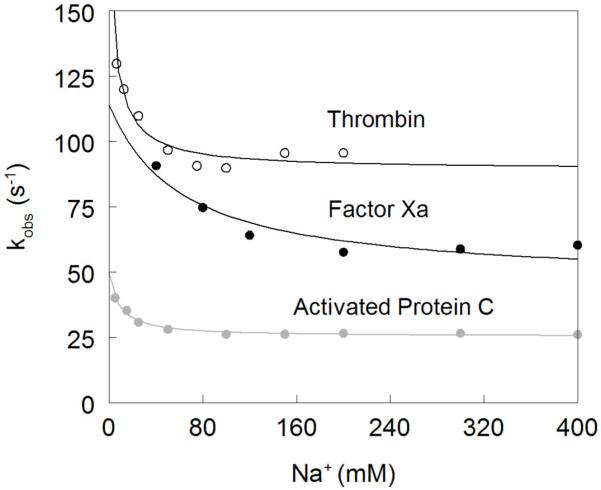
The values were obtained from analysis of the kinetic traces (see also Figure 1) and analyzed according to eq 6 in the text with best-fit parameter values listed in Table 1. Note how kobs features an inverse hyperbolic dependence on [Na+], thereby proving the existence of the E*-E equilibrium preceding Na+ binding. This demonstrates directly that the Na+-free slow form of thrombin is not a single species or a single ensemble of species, contrary to the basic assumption of alternative models of thrombin allostery 33-36. The Na+ affinity is highest for thrombin and lowest for factor Xa. The rate constants pertaining to the E*-E interconversion are fastest for thrombin and slowest for activated protein C, However, the value of the equilibrium constant r=k−r/kr is comparable for all enzymes, supporting the conclusion that the distribution of E* and E forms is conserved among different trypsin-like proteases.
