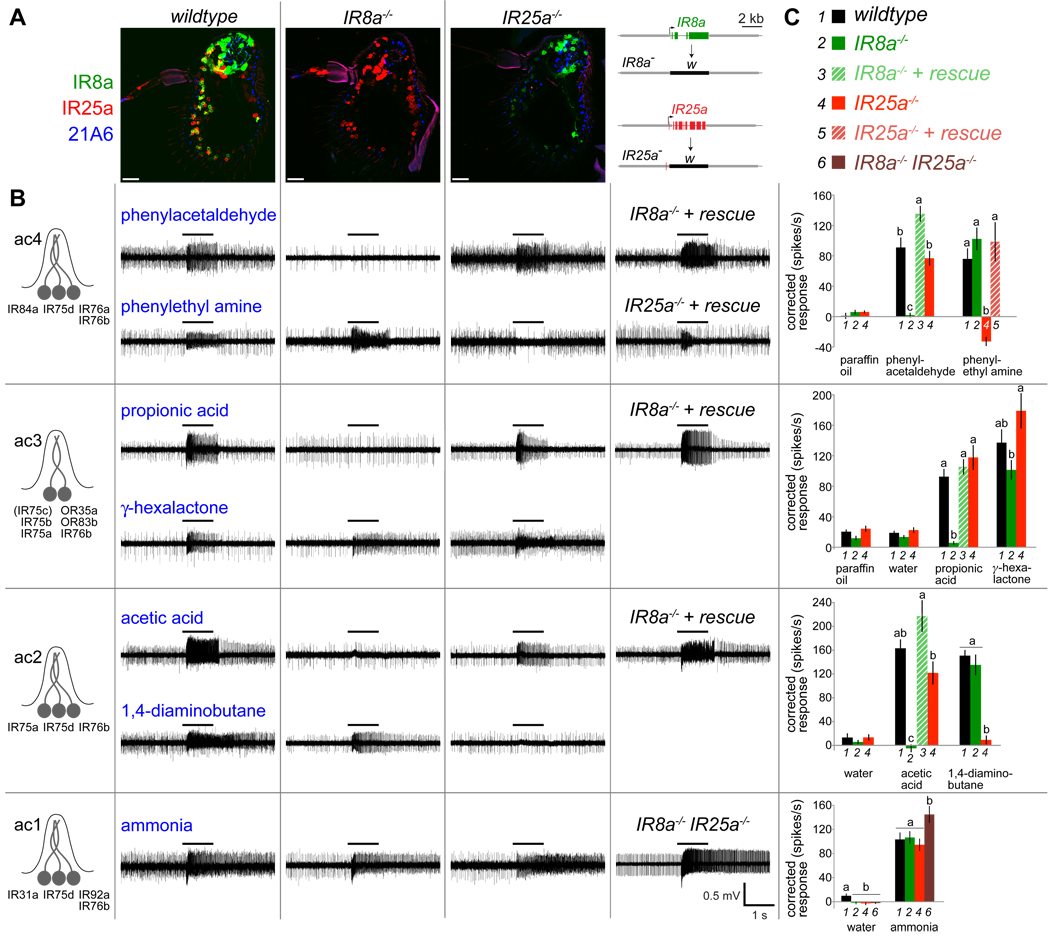
Figure 2. IR8a and IR25a are essential for odor-evoked electrophysiological responses in multiple distinct neuron classes.
(A) Immunostainings on antennal sections from wildtype (left), IR8a1 mutant (middle) and IR25a2 mutant (right) flies with IR8a (green), IR25a (red) and 21A6 (blue) antibodies. The scale bars represent 20 µm. Schematics of gene-targeted IR8a and IR25a null alleles, where the IR coding region is replaced with the white (w) reporter gene, are shown at the far right.
(B) Left: Schematic of IR expression (excluding IR8a and IR25a) in the four classes of coeloconic sensilla (ac1–ac4) (after (Benton et al., 2009)). Right: Representative traces of extracellular recordings of neuronal responses in the four coeloconic sensilla classes in wildtype (first column), IR8a1/Y hemizygous mutant (second column), IR25a2 mutant (third column), and rescue or IR8a1/Y; IR25a2 double mutant (fourth column) flies, stimulated with the indicated odors. Bars above the traces mark stimulus time (1 s). Genotypes for rescue experiments: “IR8a−/− + rescue”: IR8a1/Y;IR8a-GAL4/UAS-IR8a, “IR25a−/− + rescue”: IR25a2,IR25a-GAL4/IR25a2,UAS-IR25a.
(C) Quantification of mean neuronal responses to different odor stimuli in ac1–ac4 sensilla (± s.e.m; n=11–18 (ac4), n=8–16 (ac3), n=5–8 (ac2), n=10–13 (ac1); male flies, ≤3 sensilla/animal) in the genotypes shown in the key at the top and as detailed in (B). Paraffin oil and water are solvent controls. For individual stimuli in each sensilla, bars labeled with different letters are significantly different. ac4: phenylacetaldehyde ANOVA p<0.0001, phenylethyl amine ANOVA p<0.0001; ac3: propionic acid ANOVA p<0.0001, γ-hexalactone ANOVA p>0.011; ac2: acetic acid ANOVA p<0.0001; 1,4-diaminobutane ANOVA p<0.0001; ac1: water ANOVA p>0.0097, ammonia ANOVA p>0.022. Small, but statistically significant, variations in spike responses were observed to some odors in certain mutant backgrounds, for example ac3 γ-hexalactone responses. Although we cannot exclude a modulatory role of IR8a or IR25a in detection of these stimuli, we believe that these effects are likely to be indirect, either because of physiological changes in neighboring neurons in the same sensilla that rely absolutely on these receptors and/or technical difficulties in consistently comparing odor-evoked spike frequencies in sensilla lacking the activity of one or more neurons with those in wildtype sensilla.