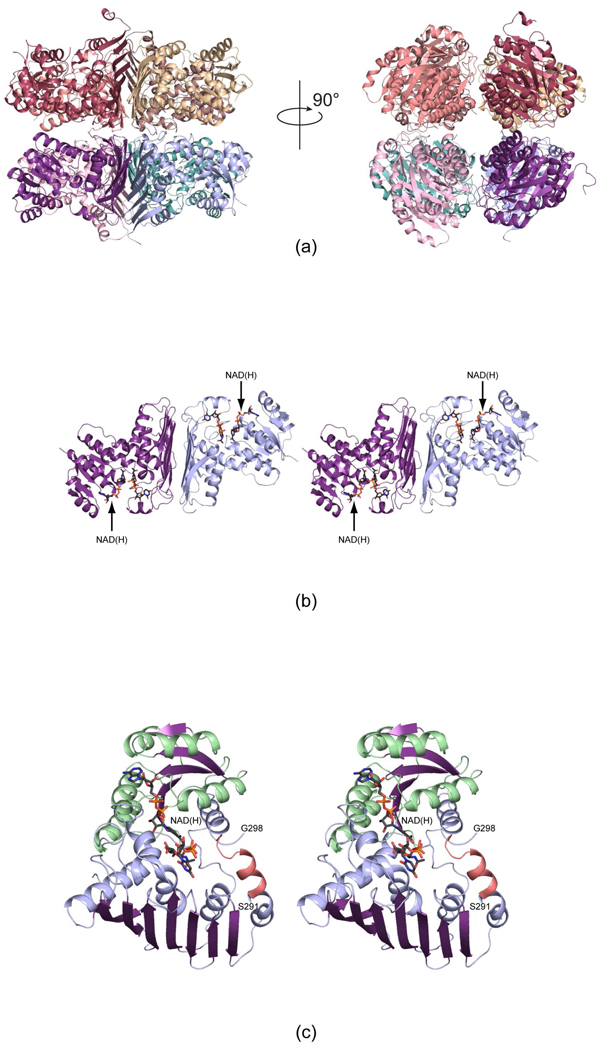
Figure 3.
The structures of WlbA from B. pertussis and C. violaceum. Both enzymes adopt octameric quaternary structures with 422 symmetry. Shown in (a) are two ribbon representations for the B. pertussis enzyme rotated 90° apart. The right hand panel shows the molecule viewed down the four-fold rotational axis. The octamers can be thought of as tetramers of dimers. One such dimer is displayed in stereo in (b). The bound NAD(H) and UDP-GlcNAcA molecules are depicted in stick representations. A close-up view of an individual subunit from the C. violaceum WlbA is presented in (c). The α-helices and β-strands for the N-terminal domain are highlighted in light green and purple, respectively, whereas those in the C-terminal domain are color-coded in light blue and purple. The α-helix displayed in salmon indicates the conformation of the region in the B. pertussis enzyme that is disordered in the C. violaceum protein.