Table 1.
Mercury-catalyzed IFCA reaction of 5a
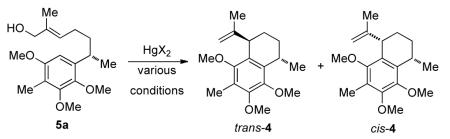
| Entry | HgX2, (equiv) | Conditions | Yield %,a (trans:cis)b |
|---|---|---|---|
| 1 | Hg(OTf)2, (0.005) | PhMe, rt, 1.5 h; 48 °C, 25 h | 40%,d 12.5:1 |
| 2 | Hg(OTf)2, (0.005) | PhMe, rt, 20 min; 60 °C, 25 h | 54%,d 8:1, |
| 3 | Hg(OTf)2, (0.005) | PhMe, 45 °C, 4 h | 54%, 9:1 |
| 4 | Hg(OTf)2, (0.005) | PhMe, 55 °C, 4 h | 50%, 9:1 |
| 5 | Hg(OTf)2, (0.005) | PhMe, 110 °C, 10 min | 34%, <9:1 |
| 6 | Hg(OTf)2,(0.01)c | PhMe, 60 °C, 30 min; 110 °C, 12 hc | 0 |
| 7 | Hg(OTf)2, (0.005) | MeCN, rt, 20 min; 70-80 °C, 19 h | 0 |
| 8 | Hg(OTf)2, (0.005) | CH2Cl2, rt, 35 min; 90 °C, 5 h | 13%,d 12.5:1 |
| 9 | Hg(OTf)2, (0.005) | ClCH2CH2Cl, rt, 35 min; 90 °C, 5 h | 27%,d 15:1 |
| 10 | Hg(SO3C4F9)2, (0.005) | PhMe, rt, 24 h; 80 °C, 15 min | e |
Yields are for isolated products unless otherwise stated.
The diastereomeric ratio was determined by analysis of the 1H NMR spectrum of the crude reaction mixture.
2,6-di-tert-butylpyridine was added.
Crude yield.
A complex mixture was formed.
