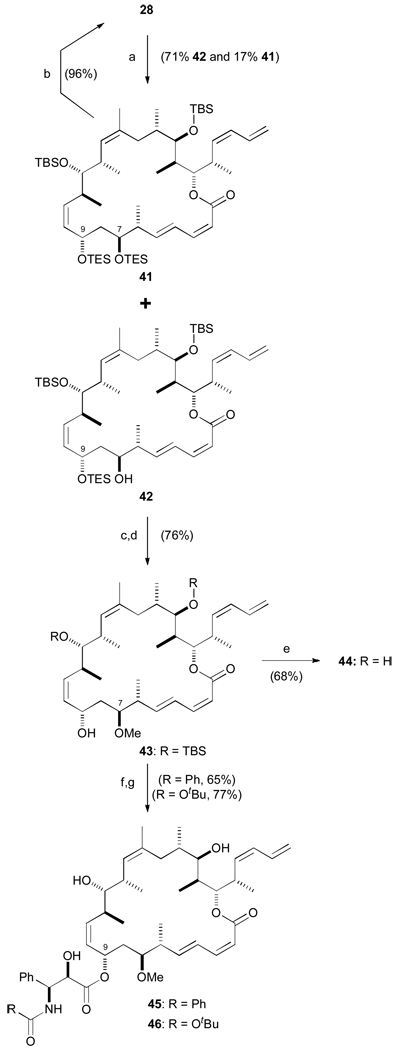
Scheme 9.
Completion of C7-methoxy analogues 44–46. a) TESOTf, 2,6-lutidine, CH2Cl2, −98 °C, 90 min; b) PPTS, MeOH / CH2Cl2, 0 → 20 °C, 2 h; c) Me3O·BF4, Proton Sponge, CH2Cl2, 20 °C, 90 min; d) PPTS, MeOH / CH2Cl2, 0 → 20 °C, 2 h; e) HF·py, pyridine, THF, 0 → 20 °C, 3 d; f) NaHMDS, THF, −78 °C, 10 min; 29 or 30, −78 → 0 °C; g) HF·py, pyridine, THF, 0 → 20 °C, 3 d. TESOTf = triethylsilyl trifluoromethanesulfonate.