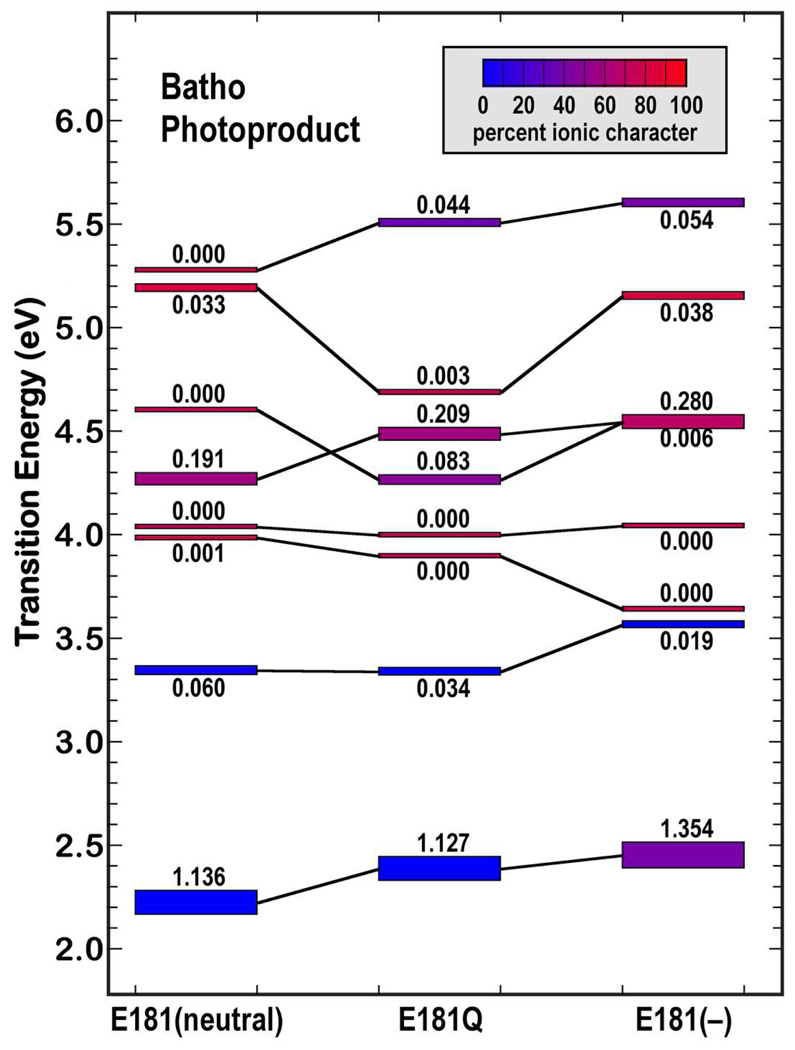
Figure 3.
Level ordering of the low-lying excited singlet states of bathorhodopsin based on SAC-CI molecular orbital theory for three cases: Glu-181 neutral (left), E181Q (middle) and Glu-181 negatively charged (right). The calculations included the 190 highest energy occupied molecular orbitals and the 190 lowest energy unoccupied molecular orbitals, with single and double excitation configuration interaction based on level three (maximum CISD) selection (36,100 singles and roughly 600,000 doubles). The covalent versus ionic character of the state is indicated by the color of the state marker and varies from blue (covalent) to red (ionic) based on the scale shown at top left. The oscillator strength of the electronic transition from the ground state is written directly above or below the state marker.