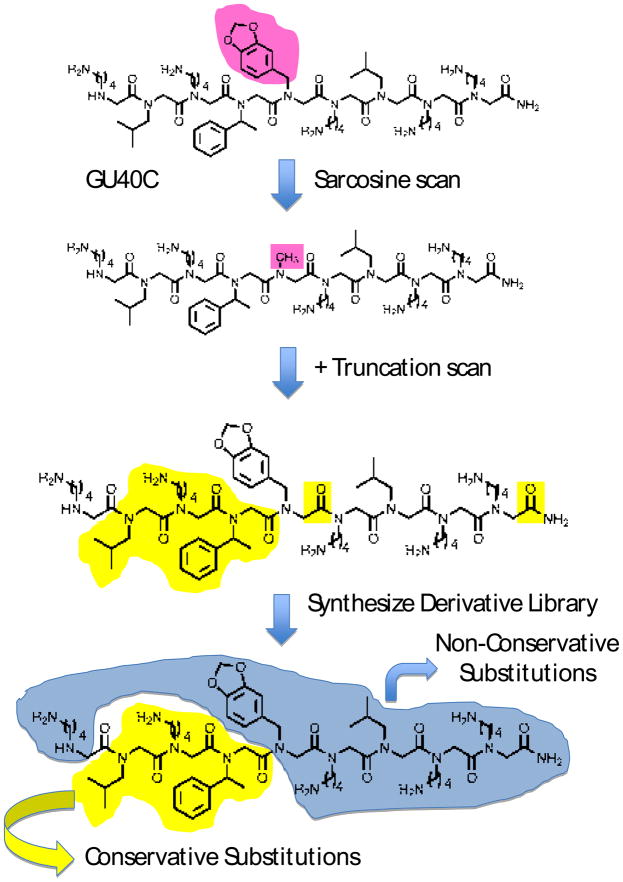
Figure 4.
Work flow for the optimization of a protein-binding peptoid using GU40C4 as an example. First, the side chains important for binding the protein are identified by synthesizing derivatives of the hit in which a methyl group is substituted for one of the side chains (for example at the position highlighted in pink). These derivatives are tested for binding to the protein target. Once the critical positions are identified, a putative minimal pharmacophore consisting of only these residues is synthesized and tested for binding (not shown). If it does not bind, truncated derivatives of the hit are made and tested for binding. In this way, the side chains and main chain residues of GU40C highlighted in yellow were found to be important for VEGFR2 binding. To identify improved derivatives of GU40C, new libraries will be created using amines different than those employed in the synthesis (see Fig. 4) of the original library. At the positions found to be important for binding, amines with structures similar, but different to that found in GU40C will be employed in an attempt to optimize the fit of these side chains with the protein pocket they contact. At positions found not to be important for binding, amines drastically different than those employed in the synthesis of the original library will be employed in an attempt to pick up new, productive peptoid-protein contacts. The derivative library will be screened again under more demanding conditions.