Figure 1.
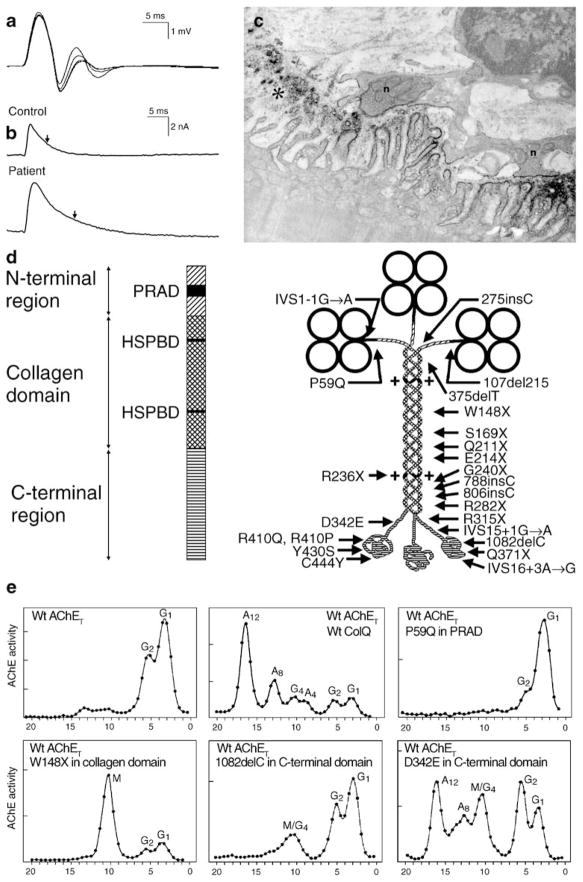
CMS caused by defects in AChE. a Repetitive CMAPs are generated by prolonged synaptic potentials that outlast the absolute refractory period of the muscle fiber. b MEPCs recorded from a normal and an AChE-deficient patient EP. Vertical arrows indicate decay time constants. The patient MEPC decays slowly as ACh repeatedly binds to AChRs before it exits the synaptic space by diffusion; the MEPC amplitude is reduced owing to the associated EP myopathy. c AChE-deficient EP region. AChR is localized with peroxidase-labeled α-bungarotoxin. Numerous junctional folds are degenerating, shedding their terminal expansions into the synaptic space. The nerve terminals are abnormally small and cover only a fraction of the postsynaptic region. d Schematic diagram showing domains of a ColQ strand and components of the A12 species of asymmetric AChE with 24 identified ColQ mutations. e Clockwise from upper left density gradient ultracentrifugation profiles of extracts of COS cells transfected with wild-type AChET, wild-type AChET and wild-type COLQ, wild-type AChET and the P59Q PRAD domain mutant of COLQ, wild-type AChET and the W148X collagen domain mutant of COLQ; wild-type AChET and the 1082delC C-terminal domain mutant of COLQ; and wild-type AChET and the D342E C-terminal domain mutant of COLQ. Note that the asymmetric forms of AChE appear only after transfection with the C-terminal D342E mutant of COLQ. AChE acetylcholinesterase, MEPC miniature EP current, HSPBD heparan sulfate proteoglycan binding domain, PRAD proline-rich attachment domain, Wt wild type, G globular moiety of AChET, A asymmetric moiety of AChE, M mutant peak. (Reproduced by permission from Engel et al. 2003b)