Figure 1. Control of Npt2a by FGF23.
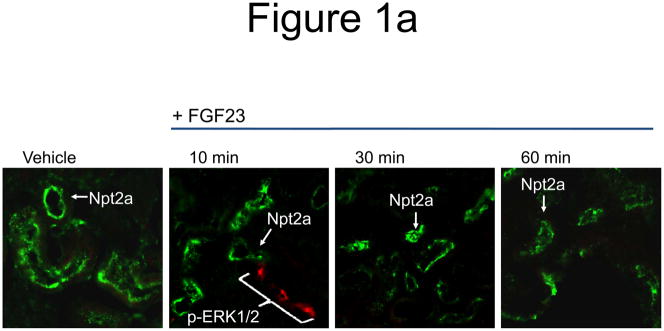
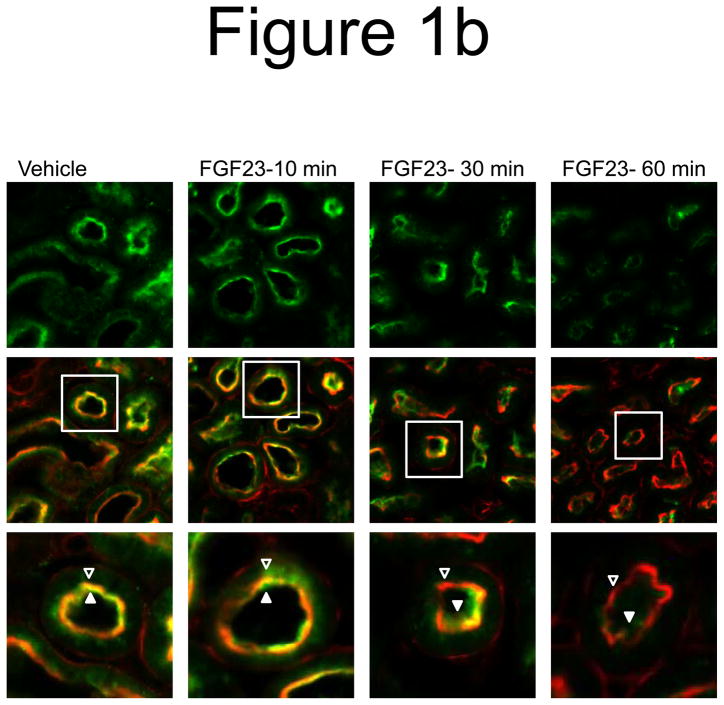
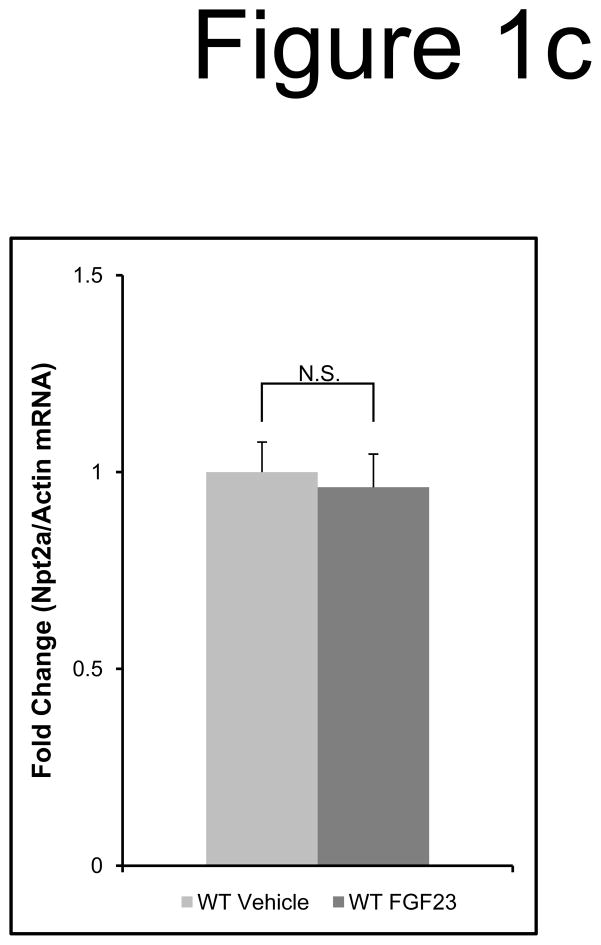
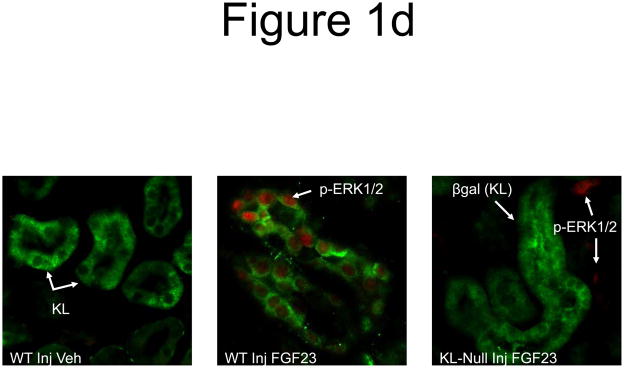
a) Mice were injected with FGF23 or vehicle for 10, 30, or 60 minutes. Under standard staining conditions Npt2a expression was tested. Npt2a was reduced 30–60 minutes following injection with FGF23 (top row). Sections were co-stained with actin (red), to mark the apical membrane, and co-localization of Npt2a in the apical membrane was detected (yellow). By 60 minutes Npt2a was not detected in the apical membrane (middle row). Higher magnification of the proximal tubule shows the progressive removal of Npt2a from the apical membrane (bottom row); b) Sections were co-stained for Npt2a and p ERK1/2. p-ERK1/2 activity was absent from the vehicle injected animals. p-ERK1/2 was confirmed in the 10 min animals and localization was distinct from Npt2a expression in the PT; no p-ERK1/2 activity was detected in the PT at any time point tested. At the 30 and 60 min time points, Npt2a staining was not detected as reduced (as in Figure 1a) due to the effects of signal amplification for Npt2a and pERK1/2. c) The reduction of Npt2a at 60 min was independent of transcription, as no change in Npt2a mRNA was detected (P=0.7). d) WT or KL-null mice were injected with vehicle or 10 μg of FGF23 (WT vehicle, left panel; WT FGF23, middle panel) to test for renal MAPK activity. In the KL-null animals, the LacZ gene is inserted into the Klotho locus, which enabledβ-galactosidase (βgal) immunofluorescence to be used as a surrogate marker for KL (green, right panel). Following FGF23 delivery to KL-null mice, p-ERK1/2 activity was absent from the DCT labeled with β-gal (KL) (right panel). As confirmed, strong p-ERK1/2 activity co-localized with KL in WT animals injected with FGF23 (middle).