Tufts CTSI Strategic Focus on Impact on Clinical Practice and Health Policy
National Institutes of Health (NIH) Clinical and Translational Science Awards (CTSAs) are intended to help transform the biomedical research enterprise to be more focused on, and effective in, improving the health of the public. 1 The focus is on translation into health impact: from bench research to the patient bedside (the first translational step, T1), from bedside research to clinical practice (T2), and from practice into overall public benefit and policy (T3 and T4), and back across each step, as well.
To maximize the impact of Tufts CTSI, based on the strengths and passions of Tufts and its partners, our focus is on impacting real‐world clinical practice and policy.
This ambitious conceptual framework is clear, but will CTSAs have real impact on health? To maximize chances for impact, each CTSA must deploy its resources strategically in a way that leverages its institution’s strengths, resources, and passions.
This focus is not new at Tufts. Thirty years ago the Tufts Sackler School of Graduate Biomedical Sciences was founded with a mission focused on interdisciplinary approaches that integrate basic and clinical sciences. At that same time, in the adjacent Tufts Medical Center with its major bench‐to‐bedside program, programs using new analytical methods arose that were directed at impact on clinical care and policy. The Division of Clinical Decision Making in the Department of Medicine started the use decision analysis, decision science, and cost‐effectiveness analysis for clinical and policy issues, and these were expanded with the later addition of the Center for Evaluation of Value and Risk. The Center for Cardiovascular Health Services Research developed “predictive instruments” as decision support for diagnosis and treatment of individual patients, and then tested them in large clinical effectiveness trials. The Health Institute, formed by the leaders of the landmark Medical Outcomes Study, showed the power of merging clinical and social sciences in outcomes research. Soon after, pioneers in clinical trial design, meta‐analysis, and evidence‐based medicine formed the Center for Clinical Evidence Synthesis. These programs were committed to training, and in 1999 we established the nation’s first M.S./Ph.D. Clinical Research Graduate Program based in a biomedical graduate school and hospital (Tufts Sackler School and Tufts Medical Center). This research and training environment with a focus on measurable impact on health provided the perspective and strengths for Tufts CTSI.
Comparative Effectiveness Research as a Focus on Impact on Clinical Care and Policy
These programs include what is now referred to as comparative effectiveness research (CER), which has a focus on informing real‐world clinical care and health policy. Tufts CTSI is committed to a broader spectrum of research than only that included in CER, but this CER focus on real‐world impact suffuses Tufts CTSI. The definitions of CER from the Institute of Medicine (IOM) 2 and the Congressionally mandated Federal Coordinating Council for CER (FCC‐CER) 3 emphasize these objectives. The IOM defined CER as:
The generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care at both the individual and population levels.
As illustrated in Figure 1 (by Joseph Lau and David Kent), CER represents a portion of the overall translational chain from bench‐to‐bedside, bedside‐to‐practice, and practice to public benefit and policy. The chain generates information for real‐world use, and also re‐informs earlier steps. This figure also emphasizes the need for continual interaction with stakeholders, including academic medical centers, industry, and community partners, depicted by the concentric ovals. To do this, Tufts CTSI has promoted participatory models of research as articulated by the NIH, 4 the IOM, 5 and the Centers for Disease Control and Prevention (CDC), that include efforts to address the disproportionate burden of chronic diseases among urban residents. 6 , 7 , 8
Figure 1.
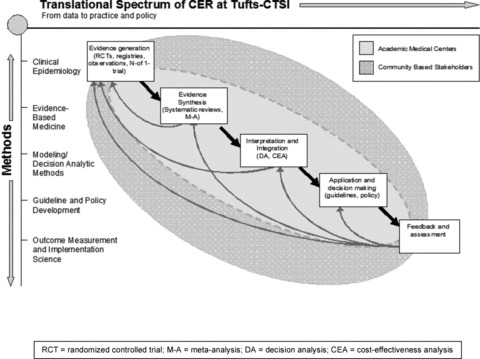
Translational spectrum of CER at Tufts‐CTSI.
As outlined in the CTSA Consortium White Paper on CER 4 there are many reasons CTSAs are well suited for facilitating CER, including their extensive multidisciplinary clinical research infrastructures, their educational and training programs, and their links to communities, practice networks, research networks, and national consortia. Additionally, to truly advance CER and to have impact on health by leveraging all types of translational research, a CTSA must have a conceptual focus on impact and the expertise to drive this. Reflecting this, in addition to the usual CTSA components, Tufts CTSI has four special components: Evidence‐Based Medicine, Genetics and Genomics Analysis, Predictive Medicine, and Therapeutics Development and Implementation. Translation into health impact is also emphasized in the components Community Engagement, the Design and Data Resources Center, and Education and Training and Career Development. All components are integrated and accessed through a single “Tufts CTSI Portal” that provides support for research across the T1‐T2‐T3‐T4 spectrum. This fusion introduces the CER real‐world impact focus to those who might otherwise not be exposed to this perspective.
Examples of Tufts CTSI Use of CER Methods and Focus on Health Impact
Oropharyngeal cancer
How the perspective and methods of CER influence all Tufts CTSI research support is exemplified by integration of our Predictive Medicine component with the other components. In late 2009, Tufts CTSI created a broad collaboration of scientists to tackle an important clinical problem in the community close to the Tufts Health Sciences Campus in Boston that includes a large Asian population. This clinical problem is cancer of the oral cavity and oropharynx, illnesses diagnosed in 35,000 Americans in 2009, and these neoplasms allow a 5‐year survival of only 56%, a figure that has shown no improvement in decades. These illness are disfiguring, very difficult to treat, and notably prevalent in the Asian community. The study fostered by the Tufts CTSI was designed to efficiently differentiate benign from malignant (or premalignant) oral lesions, a goal long out of reach. To accomplish this the Tufts CTSI convened basic scientists from Tufts and CTSI member Northeastern University and a local engineering firm that had already published data showing that infrared spectrophotometry can differentiate benign and malignant cells from the uterine cervix; such cells look alike to experienced pathologists when studied by light microscopy. In the oral cancer study that has been designed by our CTSI, these fiber optic‐based tools are being adapted as probes to develop a point of care device for oral cancer screening. Participants include oral pathologists in the Tufts University Schools of Medicine and Dental Medicine, pathologists at Tufts Medical Center, internists, Northeastern University chemists and biophysicists, and the engineering firm as mentioned earlier. Study design and method validation were carried out by the CTSI’s Predictive Medicine Component and its Design and Data Resource Center. It is highly unlikely that this collaboration among four academic and clinical institutions could have developed without facilitation by the CTSI.
Research training for community members
Having an impact on real‐world health problems requires a range of academic, clinical, community, and industry participants. The 43 Tufts CTSI organizations (see http://www.tuftsctsi.org) include 11 Schools of Tufts University, nine Tufts affiliated hospitals across New England, three academic partners, a dozen community‐based organizations, and eight industry for‐ or non‐profit partners. The opportunities are illustrated by an administrative supplement entitled “Building Your Capacity (BYC)” recently received by Tufts CTSI to build capacity among 20 community‐based organizations or clinics to promote bi‐directional research. Other community engagement leaders engaged in this project include representatives from the CTSA sites at Harvard, Boston, Columbia, and Northwestern universities. Enrolled in six training sessions, community research fellows are learning about how to participate in community engaged research and academic‐community research partnerships. As part of this program, fellows will be paired with a research consultant from the BYC Steering Committee who will support them in the development of a research project plan. The pedagogical framework we are using for these training sessions is rooted in best practice in educational research. This framework includes a focus on student–teacher relationships, community‐centered learning, building social capital among participants, and participatory learning. Some of the participating community groups are focused on diseases, some on neighborhoods, while others focus on social determinants of health, such as poverty, housing, and violence. Interestingly, although research has not typically been a part of the mission of many of these community‐based agencies we have found them to be highly committed participants that want to be proactive in addressing unmet needs in the community, and in making Tufts research responsive to the urgent needs among their constituents. Community agencies also clearly want the research originating in the CTSI or in the community groups to be more evidence based. Consistent with the recommendations of the IOM and FCC‐CER CER reports, Tufts CTSI is partnering to develop the capacity to fully involve the public and clinical practice communities as we generate, prioritize, and develop CER questions and research protocols. A natural progression at the local level will involve interpreting CER results and implementing them in practice in Tufts CTSI member hospitals and practice networks, communities, through public health agencies, and ultimately, as health care policy.
Contributing to CTSA Consortium CER development
Another example of our ongoing efforts has been our work across the CTSA Consortium to help build a national network and efficient infrastructure for conducting CER. In 2008, Tufts CTSI hosted a meeting on CER that was open to all CTSAs and other stakeholders; this led to the development of a CTSA CER Interest Group, and then in 2009 to the inclusion of CER in the CTSA Consortium Strategic Goals. The CER Strategic Goal Committee (SGC), led by the Tufts CTSI Principal Investigator, has produced a White Paper on possible roles for CTSAs in CER, 9 a report on the CTSA Consortium’s CER needs and capacities, a report on CER methods, and a report on CER workforce development and training. Wide interest in this work led to the formation of a CTSA Consortium CER Key Function Committee (KFC), which includes all CTSAs nationally. Tufts CTSI considers part of its focus on impact to continue to work to support these national CTSA efforts.
Policy advising on the role of translational research in national health care reform
Impact on clinical and health policy, a focus of CER, is also seen as integral to the translational chain at Tufts CTSI. Policy work by Tufts CTSI faculty from the Tufts Medical Center and partners at Brandeis and RAND have focused on the incorporation of CER as well as other translational research findings into policy initiatives both locally and nationally. Additionally, we have worked with the Senate Health Education and Labor Committee under the Chairmanship of the late Massachusetts Senator Kennedy, with other Congressional Offices, and with the Administration in policy formation as a way to translate research results into better care and health for all Americans.
Translational research training initiatives with a focus on health impact
The best way to maximize research impact on health care and policy is to train others who can continue and expand such work. Thus we recently reframed our M.S./Ph.D. Clinical and Translational Science Graduate Program at Tufts to emphasize translational research across the spectrum in its three Degree Concentrations: Clinical Investigation (focusing on translational steps T1‐T2); Evidence‐Based Effectiveness Research (T2‐T3); and Health Services and Health Policy Research (T3‐T4). The primary focus is training groundbreaking translational investigators, most often postgraduate fellows or junior faculty, but for those desiring less than the 2 or more years required for an M.S. or Ph.D., Tufts CTSI offers a 1‐year Certificate Program. Also, individual classes and seminars that teach specific skills for translational research are taken by clinicians, researchers, and students from other Tufts Schools. This has served as an impetus to extend provision of courses and educational programs via distance learning and development of joint programs with other Tufts graduate schools and for the public and industry. We now are increasing our distance learning and archived education resources, to increase availability and expand the impact of Tufts CTSI.
Tufts CTSI commitment to full‐spectrum translational research for impact on health
Promoting research focused on health impact requires broad based interdisciplinary, inter‐institutional, and community‐based collaborations. Tufts CTSI is well poised to do this across the T1‐T2‐T3‐T4 spectrum. At one end, the Clinical and Translational Research Center and Translational Technologies Component support researchers in early‐phase translation from basic research into clinical testing and trials with its specialized nursing support and laboratory processing of human specimens. At the other end, there are many opportunities for community‐based projects and broad expertise in CER analytic methods, including meta‐analysis, predictive modeling, decision analysis, cost‐effectiveness analysis, outcomes research, policy analysis, and a wide variety of data analysis services. Thus, while we continue to leverage our strengths in CER, we also consider its focus on the most effective treatments and health care strategies as the conceptual framework for all Tufts CTSI research. Ultimately, all treatments and health policies rest upon research from across the entire clinical and translational science spectrum. In the view of Tufts CTSI, ultimately, all translational research is about health impact.
Acknowledgments
Supported by National Center for Research Resources (NCRR), National Institutes of Health (NIH) through the Clinical and Translational Science Awards Program (CTSA) grant number: UL1 RR 025752.
Dr. Selker is Dean and Principal Investigator, Drs. Leslie, Plaut, and Wilson are Associate Directors and Co‐Principal Investigators, and Ms. Wasser is Administrative Director of Tufts Clinical and Translational Science Institute (CTSI).
references
- 1. Zerhouni E. Translational and clinical science – time for a new vision. N Engl J Med. 2005; 353(15): 1621–1622. [DOI] [PubMed] [Google Scholar]
- 2. Committee on Comparative Effectiveness Research Prioritization, Institute of Medicine (IOM) . 2009. Initial National Priorities for Comparative Effectiveness Research. Washington , DC : The National Academies Press. [Google Scholar]
- 3. US Department of Health and Human Services . 2009. Federal Coordinating Council for Comparative Effectiveness Research: Report to the President and Congress. Washington , DC : US Department of Health and Human Services. [Google Scholar]
- 4. National Institutes of Health . Report and recommendations on public trust in clinical research for the NIH director from the director’s council of public representatives (COPR). Bethesda , MD : National Institutes of Health, 2005. [Google Scholar]
- 5. Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington , DC : The National Academies, 2001. [PubMed] [Google Scholar]
- 6. Seifer S, Sisco S. Mining the challenges of CBPR for improvements in urban health (editorial notes). J Urban Health. 2006; 83(6): 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins D, Metzler M. Implementing community‐based participatory research centers in diverse urban settings. J Urban Health. 2001; 78(3): 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freeman E, Brugge D, Bennett‐Bradley W, Levy J, Rivera Carrasco E. Challenges of conducting community‐based participatory research in Boston’s neighborhoods to reduce disparities in asthma. J Urban Health. 2006; 83(6): 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Selker HP, Strom BL, Ford DE, Meltzer DO, Pauker SG, Pincus HA, Rich EC, Tompkins C, Whitlock EP. White Paper on CTSA Consortium role in facilitating comparative effectiveness research. Clin Transl Sci. 2010; 3(1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


