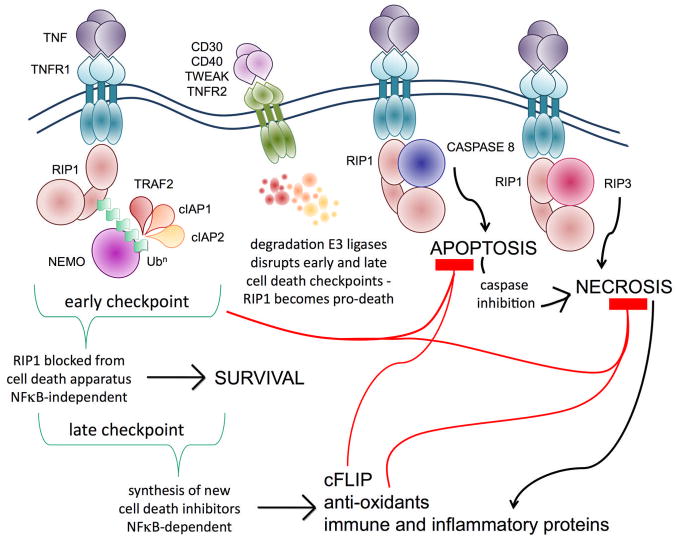
Figure 1. Control of RIP1’s pro-life and pro-death activity by ubiquitination resolves the NFκB paradox and the co-operation of TNFR signaling in immunity.
There are two cell death checkpoints in the TNFR1 pathway. In the first checkpoint, ubiquitination of RIP1 by the E3 ligases TRAF2, cIAP1 and cIAP2 hinders RIP1 from binding cell death molecules early after TNF stimulation. This checkpoint does not require new protein synthesis. In the second checkpoint, NFκB activation by ubiquitinated RIP1 leads to the production of new proteins that can block cell death, keeping cells alive in the long-term, which explains why cell survival is the default outcome of TNFR1 signaling. However, when cell death is desirable, the early checkpoint can be rapidly altered by ligation of the other TNFR family members, which trigger degradation of the E3 ligases. Non-ubiquitinated RIP1 becomes a pro-death signaling molecule and quickly binds caspase-8 to initiate apoptosis. If caspase activity is blocked, RIP1 binds RIP3 and triggers the back-up cell death pathway by programmed necrosis. Ubiquitination controls the switch between RIP1’s pro-life and pro-death activity: this resolves the NFκB paradox – ubiquitination of RIP1 prevents TNFR1 from triggering cell death until an effective pro-survival gene expression program has been implemented.
NFκB activated by TNFR1 drives expression of immune and inflammatory genes during responses to pathogens. Some pathogens attempt to block NFκB and dampen down inflammation, in order to prolong the survival of infected cells. The other TNFRs like TNFR2 can unmask the pro-death activity of RIP1 and trigger pro-inflammatory necrotic death. This co-operation between the TNFRs allows TNFR1 to circumvent the immune evasion strategies used by pathogens to block NFκB activity or apoptosis.