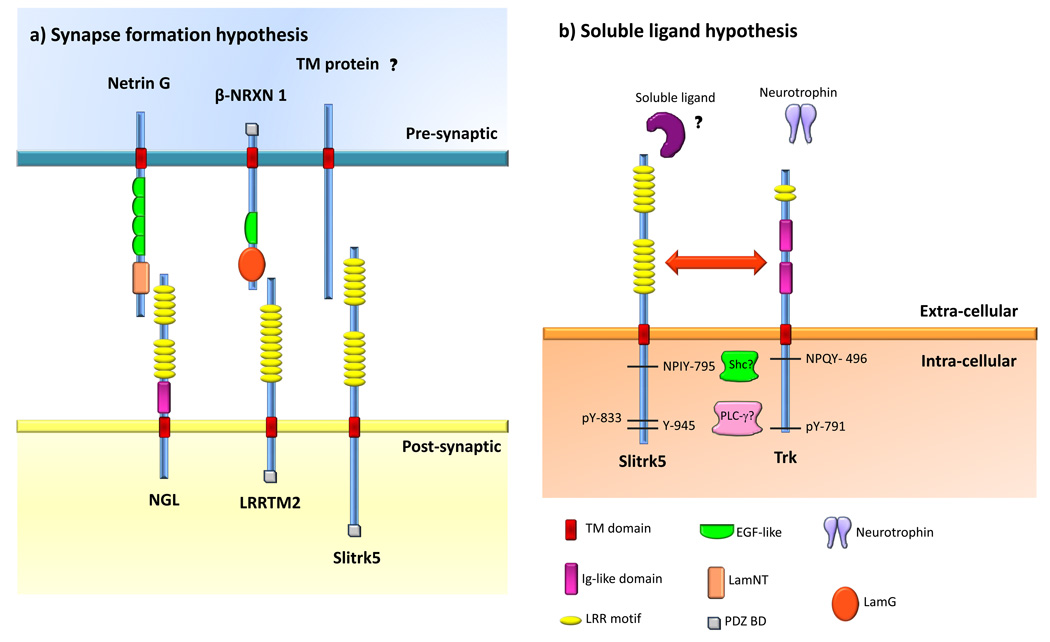
Figure 1. Possible models for the functions of Slitrks.
Slitrk5 was selected as the prototype of the Slitrk family and protein domains are shown according to the SMART database (http://smart.embl-heidelberg.de/). a) Synapse formation hypothesis for Slitrk function. According to this hypothesis, Slitrks could localize to the postsynaptic membrane and bind pre-synaptic membrane proteins. The identity of such binding partners remains unknown. This hypothesis is based on studies in culture whereby overexpression of Slitrk2 in fibroblasts induced excitatory synaptic differentiation in contacting neurons [29], as well as immunofluorescence studies demonstrating that Slitrk5 is localized at synaptic sites [28]. Other LRR-containing proteins that are known to play such a function in synapse formation are depicted. Netrin G-ligand (NGL) localizes to the post-synaptic cell, where it binds Netrin G through its LRR domains. LRRTM2 also localizes to the post-synaptic cell, where it binds both α and β Neurexins (NRXN) through its LRR domains. β-NRXN1 is shown as an example. b) Soluble ligand hypothesis. Slitrk1, 5 and 6 have been shown to play trophic roles in neurons [26–28]. Slitrk6 has been demonstrated to modulate neurotrophin signaling [26]. In PC12 cells, Slitrks modulate neurite outgrowth upon NGF treatment [1]. Thus, it is likely that Slitrks modulate RTK signaling. In this model, two possible scenarios are presented: one in which Slitrks act as receptors for soluble ligands that lead to signaling transduction events related to the neurotrophin system (ie. similar to how Trks and their neurotrophins interact). In a second scenario, Slitrks could physically interact with Trk receptors (and/or other RTKs) and directly modulate their function. The depicted Trk amino acid numbers correspond to human TrkA but similar residues and signaling components are found in TrkB and TrkC. Neurotrophin binding to Trks leads to receptor dimerization and subsequent activation and phosphorylation of their catalytic tyrosine kinase domains as well as phosphorylation of intracellular tyrosines such as the juxtamembrane NPXY domain and Y791 (in human TrkA). Y791 recruits PLC-γ. This enzyme subsequently hydrolyses phosphatidyl inositol to generate inositol tris-phosphate (IP3) and diacylglycerol (DAG), which ultimately leads to the release of Ca2+ from internal stores and protein kinase C (PKC) activation, respectively [13,16,20]. For simplicity, a single TrkA molecule is shown instead of the active dimmer. Y791 in human TrkA (and analogous tyrosines in TrkB and TrkC) show some degree of homology with the cytoplasmic tail of Slitrks [1], namely Y945 in human Slitrk5, raising the question if Slitrks are also implicated in PLC-γ signaling. Moreover, Slitrk2, 3 and 5 also contain an NPXY motif at the juxtamembrane region. Phosphorylated NPXY serves as a docking site for phosphotyrosine bindig proteins such as Shc that links the activated neurotrophin receptor to two separate intracellular signaling pathways: mitogen activated protein kinase (MAP kinase) and PI-3 kinase [17]. Slitrks might mediate similar signaling events through their conserved NPXY motif. Finally, it was found that Y833 could be phosphorylated in Slitrk5, however its function is still unknown [21]. Abbreviations: TM, transmembrane domain; Ig, immunoglobulin-like domain; LRR, leucine rich repeat; EGF, epidermal growth factor-like domain; LamNT, laminin N-terminal domain; LamG, laminin G domain.