Abstract
This study examines the incidence and predictors of pregnancy in HIV-1-discordant couples from Nairobi, Kenya. Women from 454 discordant couples were followed for up to 2 years. One-year cumulative incidence of pregnancy was 9.7%. Pregnancy rates did not differ significantly between HIV-1-infected and uninfected women (HR = 1.46). The majority of pregnancies occurred among women < 30 years old reporting a desire for future children (1-year incidence 22.2%). Pregnancy rates may be high among discordant couples, indicating desire for children may override concerns of HIV-1 transmission and increase unprotected sex, and highlighting the need to make conception safer.
Keywords: HIV, Discordant couples, Serodiscordant, Pregnancy, Predictors, Risk factor
Introduction
Pregnancy and the desire for children are common among HIV-1-discordant couples, where one partner is HIV-1-infected and other is HIV-1-uninfected, and addressing these issues poses a significant challenge [1, 2]. Pregnancy desire can override concerns of HIV-1 transmission and increase risk behavior resulting in avoidable transmissions [3], and during pregnancy the risk of acquiring HIV-1 appears to be elevated [4, 5]. While antiretroviral prophylaxis may greatly reduce the risk of mother-to-child HIV-1 transmission [6], short-course regimens initiated late in gestation do not alter risk of horizontal transmission to the uninfected male partner. Assisted reproduction techniques such as sperm washing and artificial intrauterine insemination can greatly reduce the risk of horizontal transmission [7]; however, such strategies remain out of reach for most couples affected by HIV-1 in resource-limited settings.
Defining factors associated with pregnancy in discordant couples is relevant to designing interventions to meet pregnancy desires while reducing the risk of HIV-1 transmission. Understanding the potential impact of pregnancies on the effectiveness of prevention interventions is also important. Stable HIV-1-discordant couples are increasingly common, and have become a popular study population for HIV-1 prevention trials [8, 9]. In this nested study, we assessed the incidence of pregnancy among a cohort of stable HIV-1-discordant couples in Nairobi, Kenya and identified predictors of pregnancy during the study period.
Methods
Study Participants
HIV-1-discordant couples were recruited from voluntary counseling and testing (VCT) centers in Nairobi, Kenya from September 2007 to December 2009. Participants consented to 2 years of follow-up with quarterly study visits as part of the Protective Cellular Immune Responses in HIV-1-discordant Couples study (NIH/NIAID R01 AI068431). Eligible couples reported sex ≥ 3 times in the 3 months prior to screening and planned to remain together for the duration of the study. Women could not be pregnant at enrollment, but there was no restriction on the length of time since their last pregnancy. At enrollment HIV-1-infected participants did not have a history of clinical AIDS (WHO stage IV) and were not currently on antiretroviral therapy (ART). Only women who had not had a hysterectomy and had not undergone surgical sterilization (e.g., tubal ligation) were included in this analysis. At enrollment and follow-up visits, clinical staff administered a questionnaire including sociodemographic, sexual behavior, and medical history characteristics. Questions were presented in English or Kiswahili depending on participant preference. Interviews were conducted individually to ensure confidentiality. Urine pregnancy tests (Quick Vue One Step hCG Urine Pregnancy kit, Quidel Corp., San Diego) were done at enrollment and at each quarterly follow-up visit.
Statistical Methods
Following a positive pregnancy test, the estimated conception date was defined as 2 weeks after the 1st day of the last menstrual period. The time until conception or end of follow-up was calculated for each participant and Cox proportional hazards regression was used to estimate cumulative incidence and to assess the association between participant characteristics and pregnancy-free survival time. Only time until the first pregnancy was used in the analysis, and time to subsequent pregnancies was not evaluated. A set of characteristics was selected a priori as potential correlates of pregnancy in discordant couples. Univariate associations with pregnancy were assessed for each characteristic. Age and self-reported desire for additional children at enrollment were identified a priori as potential confounders. Adjusted models were fit separately for each potential correlate, adjusting for age and desire for additional children. Additionally, the adjusted model for the association between the number of sex acts reported in the month before enrollment was also adjusted for reported unprotected sex during the same period and the use of any modern contraceptive method at enrollment. Univariate and adjusted models for the CD4 count and viral load of the woman were restricted to HIV-1-infected women, and models for the CD4 count and viral load of the partner were restricted to uninfected women. The proportional hazards assumption was tested in each model using a test of the Schoenfeld residuals.
Results
A total of 454 women in HIV-1-discordant couples had at least 1 follow-up visit, of which 293 (64.5%) were HIV-1-infected and 161 (35.5%) were uninfected. The median age was 29 years (interquartile range [IQR] 24–34 years) and the median relationship duration was 5.1 years (IQR 2.3–9.8 years). Participants had a median of 2.0 (IQR 1.0–3.0) living children and 204 (45.7%) reported a desire for additional children. Any unprotected sex in the month before enrollment was reported by 70 (17.5%) women and current modern contraceptive use (hormonal contraceptives or intrauterine device) at enrollment was reported by 92 (20.6%) women. Current modern contraceptive use was less common among those who reported a desire for future children compared to those who reported no desire for future children (15.2% vs. 24.8%). Less than primary education was reported by 112 (24.7%) women. HIV-1-infected and uninfected women were generally similar; however, the HIV-1-infected women tended to be somewhat younger and reported a shorter relationship duration (see Table 1). A larger proportion of the infected women reported a desire for additional children and a smaller proportion reported using modern contraceptives at enrollment.
Table 1.
Baseline characteristics of women in HIV-1-discordant couples enrolled in the prospective cohort study, by HIV-1 status
| Characteristic | Overall | HIV-1-infected | HIV-1-uninfected |
|---|---|---|---|
| N = 454 | N = 293 | N = 161 | |
| n (%)* | |||
| Desire additional children | 204 (45.7) | 137 (47.9) | 66 (41.5) |
| History of STI | 110 (25.3) | 77 (27.4) | 33 (21.6) |
| Number of living children | |||
| 0 | 54 (11.9) | 36 (12.3) | 18 (11.2) |
| 1–2 | 260 (57.3) | 178 (60.8) | 82 (50.9) |
| ≥3 | 140 (30.8) | 79 (27.0) | 61 (37.9) |
| Any unprotected sexa | 70 (17.5) | 48 (18.1) | 22 (16.2) |
| Current modern contraceptive useb | 92 (20.6) | 53 (18.4) | 39 (24.5) |
| Oral | 13 (2.9) | 8 (2.8) | 5 (3.2) |
| Injectable | 66 (14.9) | 41 (14.3) | 25 (15.8) |
| Implants | 10 (2.3) | 4 (1.4) | 6 (3.8) |
| IUD | 3 (0.7) | 0 (0.0) | 3 (1.9) |
| < Primary school education | 112 (24.7) | 69 (23.5) | 43 (26.7) |
| Median (interquartile range) | |||
| Age | 29 (24, 34) | 28 (24, 33) | 29 (25, 34) |
| Lifetime partners | 3 (2, 4) | 3 (2, 4) | 3 (2, 3) |
| Relationship duration (years) | 5.1 (2.3, 9.8) | 4.6 (2.2, 8.4) | 5.8 (2.4, 11.2) |
| Sex acts with study partnera | 4 (2, 8) | 4 (2, 8) | 4 (1, 8) |
| CD4 count | 457 (302, 640) | – | |
| Partner’s CD4 count | – | 358 (236, 513) | |
| Log10 viral load | 4.3 (3.6, 5.0) | – | |
| Partner’s Log10 viral load | – | 4.7 (4.1, 5.3) | |
Numbers may not add to total because of missing data
In the month before enrollment
At enrollment
We observed 56 pregnancies in 561.2 woman-years of follow-up, for a rate of 10.0 pregnancies per 100 woman-years and an overall 1-year cumulative incidence of pregnancy of 9.7% (95% confidence interval [CI], 7.1–13.1%). The incidence of pregnancy declined with older age (Hazard ratio [HR] = 0.92; 95% CI, 0.88–0.96) (see Table 2). Women who reported a desire for additional children were almost 4-fold more likely to become pregnant (HR = 3.84; 95% CI, 2.12–6.93), with a 6-month cumulative incidence of 10.4% (95% CI, 6.9–15.6%) and a 1-year incidence of 17.4% (95% CI, 12.5–24.0%) compared to a 6-month incidence of 2.1% (95% CI, 0.9–5.0%) and a 1-year incidence of 3.7% (95% CI, 1.9–7.4%) among women not reporting a desire for additional children. Additionally, number of sex acts in the month before enrollment (HR = 1.08; 95% CI, 1.04–1.13) was associated with an increased pregnancy rate, and increasing relationship duration (HR = 0.90; 95% CI, 0.84–0.96) was inversely associated with pregnancy rate. In multivariate models adjusting for age and desire for additional children, only age (HR = 0.92; 95% CI, 0.88–0.96), desire for additional children (HR = 3.09; 95% CI, 1.67–5.70), and number of sex acts in the month before enrollment (HR = 1.07; 95% CI, 1.03–1.11) remained significantly associated with pregnancy.
Table 2.
Baseline correlates of pregnancy
| Characteristic | HR | 95% CI | aHRa | 95% CI |
|---|---|---|---|---|
| HIV-1-infected (compared to uninfected) | 1.46 | (0.81–2.64) | 1.24 | (0.68–2.25) |
| Desire additional children | 3.84 | (2.12–6.93) | 3.09 | (1.67–5.70) |
| Age (per year) | 0.92 | (0.88–0.96) | 0.94 | (0.90–0.99) |
| Relationship duration (per year) | 0.90 | (0.84–0.96) | 0.96 | (0.89–1.03) |
| Number of living children | ||||
| 0 | 1 | Reference | 1 | Reference |
| 1–2 | 0.90 | (0.42–1.93) | 1.29 | (0.60–2.80) |
| ≥3 | 0.45 | (0.18–1.13) | 1.35 | (0.48–3.81) |
| Sex acts with study partnerc | 1.08 | (1.04–1.13) | 1.07b | (1.03–1.11) |
| Any unprotected sexc | 1.48 | (0.78–2.81) | 1.32 | (0.69–2.50) |
| Current modern contraceptive used | 0.47 | (0.20–1.10) | 0.56 | (0.24–1.31) |
| < Primary school education | 0.71 | (0.37–1.37) | 1.02 | (0.52–1.98) |
| HIV-1-infected women | ||||
| CD4 count (per 100 cells/µl) | 1.10 | (0.98–1.23) | 1.10 | (0.97–1.24) |
| Log10 viral load | 0.96 | (0.69–1.34) | 0.99 | (0.70–1.39) |
| HIV-1-uninfected women | ||||
| Partner’s CD4 count (per 100 cells/µl) | 1.15 | (0.91–1.45) | 1.06 | (0.84–1.34) |
| Partner’s Log10 viral load | 0.82 | (0.46–1.47) | 0.75 | (0.36–1.54) |
Hazard ratios (HR) are based on Cox proportional hazards regression models. Separate multivariate models were fit for each correlate adjusting for age and desire for future children at enrollment
Numbers in bold face indicate p < 0.05
Adjusted for age and desire for future children at enrollment
Adjusted for age, desire for future children, any unprotected sex, and modern contraceptive use
In the month before enrollment
At enrollment; includes hormonal contraceptive use or intrauterine device
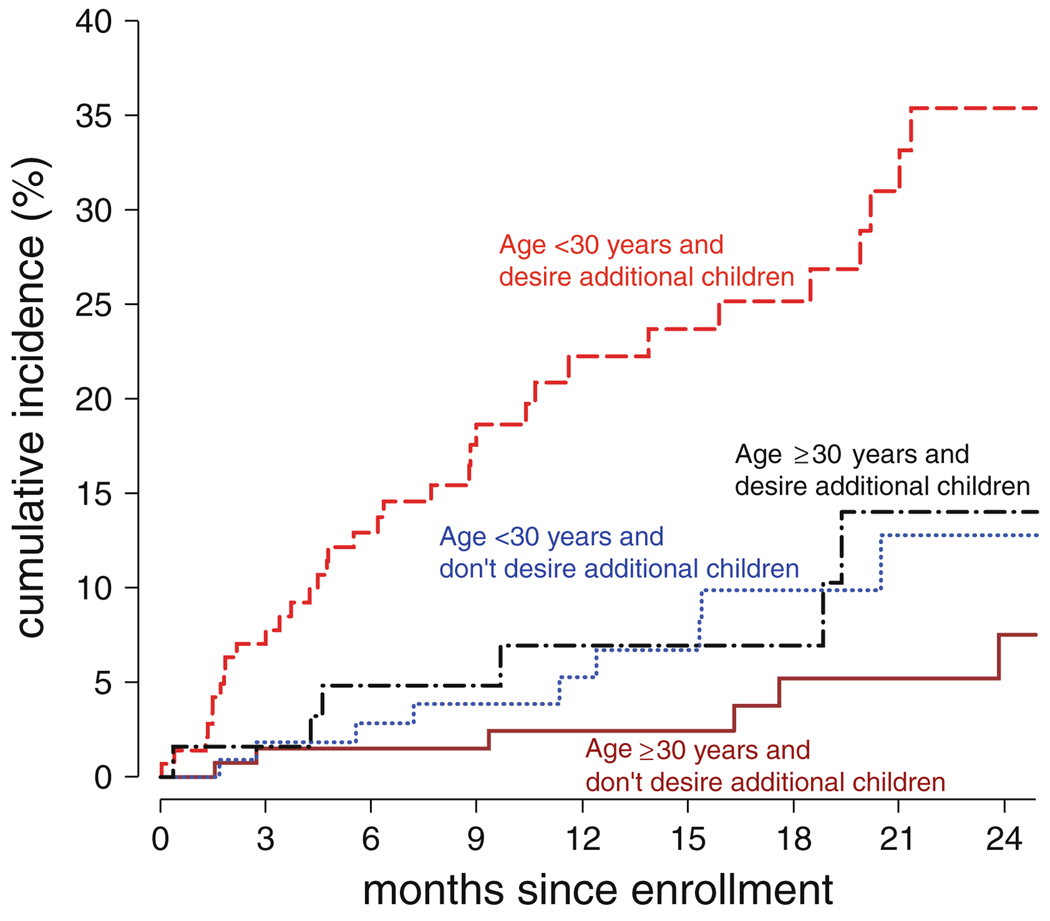
An interaction model was fit to compare pregnancy incidence between groups defined by age (<30 vs. ≥30) and desire for additional children (see Table 3). From this model, the elevated likelihood of pregnancy was restricted almost completely to those <30 who reported a desire for additional children (see Fig. 1).
Table 3.
Interaction between age and desire for additional children in relation to pregnancy incidence
| Age | Desire additional children | n (%) | Pregnancy ratea | HR | 95% CI |
|---|---|---|---|---|---|
| Age ≥ 30 years | No | 133 (29.8) | 3.2 | 1 | Reference |
| Age < 30 years | No | 109 (24.4) | 6.4 | 1.98 | (0.70–5.56) |
| Age ≥ 30 years | Yes | 62 (13.9) | 8.8 | 2.69 | (0.91–8.02) |
| Age < 30 years | Yes | 142 (31.8) | 22.9 | 6.94 | (2.91–16.55) |
Hazard ratios (HR) are based on a Cox proportional hazards regression model with categories for all combinations of age (<30 vs. ≥30) and self-reported desire for additional children at enrollment
Numbers in bold face indicate p < 0.05
Per 100 woman-years
Fig. 1.
Female participants in HIV-1-discordant couples were tested for pregnancy every 3 months. Estimated cumulative incidence is shown for [- - -] women age < 30 years and reporting a desire for additional children (n = 133), [–·–] women age ≥ 30 years and reporting a desire for additional children (n = 109), […] women age < 30 years and not reporting a desire for additional children (n = 62), and [—] women age ≥ 30 years and not reporting a desire for additional children (n = 142)
Discussion
We found that pregnancies were common among women in HIV-1-discordant couples, with an overall rate of 10.0 pregnancies per 100 woman-years, and we identified younger age and desire for future children as strong predictors of pregnancy, with a peak rate of 22.9 per 100 woman-years among women <30 who reported a desire for additional children. The pregnancy rate in this cohort is consistent with recent observations from Uganda that found an overall pregnancy rate of 8.2 per 100 woman-years among HIV-1-infected women following initiation of ART [10, 11], and with discordant couples from sites in South Africa, Zambia, and Zimbabwe where the average pregnancy rate was 13.2 per 100 woman-years [12]. Similarly, a study in Malawi found a pregnancy rate of 13.9 per 100 woman-years among HIV-1-infected women and 14.2 per 100 woman-years among HIV-1-uninfected women [13]. By comparison, the 2008 US Census Bureau age-specific fertility rate for Kenyan women aged 20–29 years old was 23.0 births per 100 woman-years and for women aged 30–34 the rate was 18.9 births per 100 woman-years [14]. While the overall pregnancy rate observed in this cohort is considerably lower than that of the general population, the rate among younger women who stated a desire for additional children is roughly the same as the general population. Thus, this group deserves special attention in balancing fertility desires with reducing the risk of HIV-1 transmission.
As the HIV-1 epidemic progresses, prevalence of stable discordant couples is likely to continue to increase, and the expanding availability of ART means that an increasing number of HIV-infected individuals in discordant couples will be living longer, healthier lives and will desire children. These couples will require individualized information regarding risk-reduction while trying to conceive in order to avoid heterosexual HIV-1 transmission. It is unlikely to be effective to counsel only to avoid unprotected sex, ignoring the pressure and desire to have children. Strategies are needed to modify exposure to minimize HIV-1 transmission risk while maximizing the chances for conception. These include pre- and post-exposure prophylaxis, frequent pregnancy testing to avoid unnecessary unprotected sex, and coital timing to maximize fertility [15]. We found no significant difference in pregnancy rates between HIV-1-infected and uninfected women, and in fact observed a non-significant association of higher pregnancy rates among the infected women. Successful prevention of mother-to-child transmission campaigns, combined with increasing access to ART, may be reducing concerns among infected women about transmitting HIV-1 to their infant and increasing confidence that they will be healthy enough to care for their children.
The high pregnancy rate among women who reported a desire for future children highlights the need for conception counseling to reduce HIV transmission risk while meeting the family planning desires of the couples. At the same time, our finding that about 5% of women who reported no desire for additional children became pregnant, and that only 24.8% of these women were using modern contraceptive methods other than condoms also offers an opportunity to provide family planning services within HIV programs that support discordant couples in order to prevent unintended pregnancies.
Understanding pregnancy in HIV-1-discordant couples is also important because the population effectiveness of prevention interventions, such as male circumcision, microbicides, early initiation of ART, and pre-exposure prophylaxis are dependent in part on the characteristics of the target populations, including the prevalence of risky behavior. Pregnancy rates and the relationship between pregnancy desire and HIV-1 transmission risk is unlikely to be constant between populations, or even within populations over time. Therefore, it is critical to understand these factors in terms of their population-specific context, and to interpret pregnancy in relation to the environment in which population-level interventions are planned.
Pregnancy desire is also highly relevant to the internal and external validity of clinical and intervention trials. Depending on the objectives of the study and the impact of pregnancies on study protocols, pregnancy desire should be considered in the inclusion/exclusion criteria. In this study, we identify a simple means of predicting short-term pregnancy incidence based on age and self-reported desire for children. Using these predictors, participants can be grouped into categories of pregnancy probability. This can be used to include/exclude couples with high pregnancy probability or can be used to weight recruitment to account for potential attrition due to incident pregnancies. Exclusion of couples most likely to become pregnant may be efficient if there is a concern that pregnancy will result in suspension of study protocols or withdrawal of the couple from the study. However, exclusion of such couples would also affect transmission risk and generalizability of the findings.
This study benefited from a relatively large sample size and was nested within a longitudinal study that provided a high level of retention and quality assurance. Nevertheless, some limitations exist. Participants were recruited from couples found to be discordant after testing at a couples VCT. This may have resulted in some selection bias as younger couples planning to get married or planning a pregnancy may be over-represented in this population, leading to higher pregnancy rates and differential distribution of characteristics related to pregnancy. Pregnancy testing was conducted quarterly, and it is likely that some pregnancies were detected that were subsequently miscarried early in pregnancy. Such pregnancies would likely go unrecognized or unreported in the absence of regular pregnancy testing, resulting in an estimate of the pregnancy rate that is higher than would have been found otherwise. We estimated the conception date based on the self-reported date of last menstruation. While this is a relatively reliable estimate when menstruation is regular and accurately reported, recall errors may have resulted in inaccurate conception dates. The resulting pregnancy rates may therefore be over or under estimated. However, it is unlikely that this would substantially affect the relative rates, thus the associations with the correlates of pregnancy are most likely accurate. Finally, we relied on self-reported condom use, contraceptive use, and measures of sexual activity, which may be subject to some misreporting, particularly over-reporting of condom use.
In conclusion, we found a high pregnancy rate among women in HIV-1-discordant couples and identified key predictors of pregnancy, emphasizing the need to develop prevention strategies to make conception safer in discordant couples. Furthermore, the success of future intervention programs is dependent in part on behavioral factors influencing transmission risk and uptake of the intervention, and pregnancy desire is an important mediator of many of these behaviors in discordant couples. Finally, pregnancies can significantly affect the success and generalizability of intervention trials, and should therefore be explicitly addressed when planning and conducting these trials.
Acknowledgments
We thank the couples who participated in the study. Funding for this study was provided by the NIH/NIAID research grant R01 AI068431. Funding was also provided by the UW STD/AIDS training grant, grant T32 AI07140.
Contributor Information
Brandon L. Guthrie, Email: brguth@u.washington.edu, Department of Epidemiology, University of Washington, Box 359909, 325 Ninth Avenue, Seattle, WA 98104-2499, USA.
Robert Y. Choi, Department of Medicine, University of Washington, Seattle, WA, USA
Rose Bosire, Centre for Public Health Research, Kenya Medical Research Institute, Nairobi, Kenya.
James N. Kiarie, Department of Obstetrics and Gynaecology, University of Nairobi, Nairobi, Kenya
Romel D. Mackelprang, Department of Epidemiology, University of Washington, Box 359909, 325 Ninth Avenue, Seattle, WA 98104-2499, USA
Anne Gatuguta, Department of Community Health, University of Nairobi, Nairobi, Kenya.
Grace C. John-Stewart, Department of Epidemiology, University of Washington, Box 359909, 325 Ninth Avenue, Seattle, WA 98104-2499, USA Department of Medicine, University of Washington, Seattle, WA, USA; Department of Global Health, University of Washington, Seattle, WA, USA.
Carey Farquhar, Department of Epidemiology, University of Washington, Box 359909, 325 Ninth Avenue, Seattle, WA 98104-2499, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
References
- 1.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17(5):733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 2.Myer L, Morroni C, Rebe K. Prevalence and determinants of fertility intentions of HIV-infected women and men receiving antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2007;21(4):278–285. doi: 10.1089/apc.2006.0108. [DOI] [PubMed] [Google Scholar]
- 3.Brubaker S, Bukusi EA, Odoyo J, Achando J, Okumu A, Cohen C. Pregnancy and HIV transmission among HIV discordant couples in a clinical trial in Kisumu, Kenya; 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa: 2009. [abstract WELBC105] [DOI] [PubMed] [Google Scholar]
- 4.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 5.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23(10):1255–1259. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- 6.Volmink J, Siegfried N, van der Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2007;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. doi:10.1002/14651858.CD003510.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Bujan L, Hollander L, Coudert M, et al. Safety and efficacy of sperm washing in HIV-1-serodiscordant couples where the male is infected: results from the European CREAThE network. AIDS. 2007;21(14):1909–1914. doi: 10.1097/QAD.0b013e3282703879. [DOI] [PubMed] [Google Scholar]
- 8.Lingappa JR, Kahle E, Mugo N, Mujugira A, Magaret A, et al. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the partners study. PLoS ONE. 2009;4(4):e5272. doi: 10.1371/journal.pone.0005272. doi:10.1371/journal.pone.0005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guthrie BL, de Bruyn G, Farquhar C. HIV-1-discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr HIV Res. 2007;5(4):416–429. doi: 10.2174/157016207781023992. [DOI] [PubMed] [Google Scholar]
- 10.Bunnell R, Ekwaru JP, Solberg P, Wamai N, Bikaako-Kajura W, Were W, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20(1):85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 11.Homsy J, Bunnell R, Moore D, King R, Malamba S, et al. Reproductive intentions and outcomes among women on antiretroviral therapy in rural Uganda: a prospective cohort study. PLoS ONE. 2009;4(1):e4149. doi: 10.1371/journal.pone.0004149. doi:10.1371/journal.pone.0004149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid SE, Dai JY, Wang J, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr. 2010;53(5):606–613. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taulo F, Berry M, Tsui A, et al. Fertility intentions of HIV-1 infected and uninfected women in Malawi: a longitudinal study. AIDS Behav. 2009;13 Suppl 1:20–27. doi: 10.1007/s10461-009-9547-9. [DOI] [PubMed] [Google Scholar]
- 14.US Census Bureau IPC, Global population profile. International database: age-specific fertility rates and selected derived measures. 2010. [Google Scholar]
- 15.Matthews LT, Mukherjee JS. Strategies for harm reduction among HIV-affected couples who want to conceive. AIDS Behav. 2009;13 Suppl 1:5–11. doi: 10.1007/s10461-009-9551-0. [DOI] [PubMed] [Google Scholar]